Metabolomic and kinetic investigations on the electricity-aided production of butanol by Clostridium pasteurianum strains
- PMID: 33716617
- PMCID: PMC7923553
- DOI: 10.1002/elsc.202000035
Metabolomic and kinetic investigations on the electricity-aided production of butanol by Clostridium pasteurianum strains
Abstract
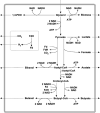
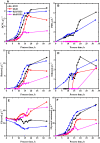
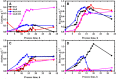
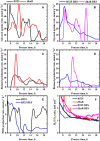
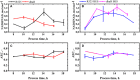
In this contribution, we studied the effect of electro-fermentation on the butanol production of Clostridium pasteurianum strains by a targeted metabolomics approach. Two strains were examined: an electrocompetent wild type strain (R525) and a mutant strain (dhaB mutant) lacking formation of 1,3-propanediol (PDO). The dhaB-negative strain was able to grow on glycerol without formation of PDO, but displayed a high initial intracellular NADH/NAD ratio which was lowered subsequently by upregulation of the butanol production pathway. Both strains showed a 3-5 fold increase of the intracellular NADH/NAD ratio when exposed to cathodic current in a bioelectrochemical system (BES). This drove an activation of the butanol pathway and resulted in a higher molar butanol to PDO ratio for the R525 strain. Nonetheless, macroscopic electron balances suggest that no significant amount of electrons derived from the BES was harvested by the cells. Overall, this work points out that electro-fermentation can be used to trigger metabolic pathways and improve product formation, even when the used microbe cannot be considered electroactive. Accordingly, further studies are required to unveil the underlying (regulatory) mechanisms.
Keywords: BES; Clostridium pasteurianum; butanol production; electro‐fermentation; redox metabolism.
© 2020 The Authors. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures

References
-
- Malaviya, A. , Jang, Y.‐S. , Lee, S. Y. , Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl. Microbiol. Biotechnol. 2012, 93, 1485–1494. - PubMed
-
- Sarchami, T. , Johnson, E. , Rehmann, L. , Optimization of fermentation condition favoring butanol production from glycerol by Clostridium pasteurianum DSM 525. Bioresour. Technol. 2016, 208, 73–80. - PubMed
-
- Zeng, A.‐P. , Biebl, H. , Bulk chemicals from biotechnology: the case of 1,3‐propanediol production and the new trends, in: Scheper, T. , Babel, W. , Blanch, H. W. , Cooney, C. L. et al. (Eds.), Tools and Applications of Biochemical Engineering Science. Advances in Biochemical Engineering/Biotechnology, Springer Berlin Heidelberg, Berlin, Heidelberg: 2002, pp. 239–259. - PubMed
-
- Lidia, S.‐R. , Stanisław, B. , Production of dihydroxyacetone from an aqueous solution of glycerol in the reaction catalyzed by an immobilized cell preparation of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur. Food Res. Technol. 2012, 235, 1125–1132.
-
- Song, H. , Lee, S. Y. , Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006, 39, 352–361.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
