Protroca: A Noninterventional Study on Prophylactic Lipegfilgrastim against Chemotherapy-Induced Neutropenia in Nonselected Breast Cancer Patients
- PMID: 33716632
- PMCID: PMC7923841
- DOI: 10.1159/000506622
Protroca: A Noninterventional Study on Prophylactic Lipegfilgrastim against Chemotherapy-Induced Neutropenia in Nonselected Breast Cancer Patients
Abstract
Background: Protroca evaluated the efficacy and safety of primary and secondary prophylaxis of neutropenia with lipegfilgrastim (Lonquex®) in breast cancer patients receiving neoadjuvant or adjuvant chemotherapy (CT).
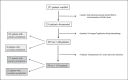
Patients and methods: Of the 255 patients enrolled, 248 patients were evaluable for the intent-to-treat (ITT) and 194 patients for the per-protocol set. Primary and secondary end points after lipegfilgrastim treatment were assessed.
Results: Nine patients of the ITT set receiving lipegfilgrastim as primary prophylaxis (n = 222) had febrile neutropenia of grade 3-4 (5 patients) or infection of grade 3-4 (4 patients); 1/26 of those receiving secondary prophylaxis had an event. Dose reductions were performed in 9.5% of the patients. Postponement of cancer CT cycles for >3 days occurred in <15% of patients; 10.8% (92/851 AEs) and 8% (2/25 SAEs) of documented adverse events and serious adverse events, respectively, were related to lipegfilgrastim.
Conclusions: Application of lipegfilgrastim was effective as primary and secondary prophylaxis in the prevention of CT-induced neutropenia in breast cancer.
Keywords: Breast cancer; Chemotherapy; Febrile neutropenia prophylaxis; Lipegfilgrastim.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
Rachel Wuerstlein received personal fees/travel support from Agendia, Amgen, Astra Zeneca, Boehringer Ingelheim, Carl Zeiss, Celgene, Daiichi-Sankyo, Esai, Genomic Health, Glaxo Smith Kline, Hexal, Lilly, MSD, Mundipharma, NanoString Technologies, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnology, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, and Teva. Nadia Harbeck received personal fees (lectures and/or consulting) and research funding from Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis, Pfizer, Roche, AstraZeneca, Hexal, Roche/Genentech, Sandoz, and Boehringer Ingelheim (Inst). Ulrike Nitz received personal fees from Genomic Health and Roche. Oleg Gluz received personal fees/travel support from Genomic Health, NanoString Technologies, Roche, Celgene, and Teva. Eva-Maria Grischke received personal fees from Roche. Dirk Forstmeyer received personal fees (speaker's bureau and consulting) from Pfizer, Novartis, Roche, and MSD.
Figures
References
-
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006 May;106((10)):2258–66. - PubMed
-
- Pfeil AM, Allcott K, Pettengell R, von Minckwitz G, Schwenkglenks M, Szabo Z. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer. 2015 Feb;23((2)):525–45. - PubMed
-
- Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003 Dec;98((11)):2402–9. - PubMed
-
- Wolff D, Culakova E, Poniewierski MS, Lyman GH, Dale DC, Crawford J, Awareness of Neutropenia in Chemotherapy Study Group Predictors of chemotherapy-induced neutropenia and its complications: results from a prospective nationwide registry. J Support Oncol. 2005 Nov-Dec;3((6 Suppl 4)):24–5. - PubMed
-
- Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008 Feb;6((2)):109–18. - PubMed
LinkOut - more resources
Full Text Sources

