Parkinson's Disease: Can Targeting Inflammation Be an Effective Neuroprotective Strategy?
- PMID: 33716638
- PMCID: PMC7946840
- DOI: 10.3389/fnins.2020.580311
Parkinson's Disease: Can Targeting Inflammation Be an Effective Neuroprotective Strategy?
Abstract
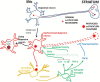
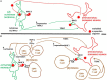
The reason why dopamine neurons die in Parkinson's disease remains largely unknown. Emerging evidence points to a role for brain inflammation in neurodegeneration. Essential questions are whether brain inflammation happens sufficiently early so that interfering with this process can be expected to slow down neuronal death and whether the contribution from inflammation is large enough so that anti-inflammatory agents can be expected to work. Here I discuss data from human PD studies indicating that brain inflammation is an early event in PD. I also discuss the role of T-lymphocytes and peripheral inflammation for neurodegeneration. I critically discuss the failure of clinical trials targeting inflammation in PD.
Keywords: T-cells; alpha-synuclein; brain; cervical lymph node; gut; microglia.
Copyright © 2021 Gundersen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Acuña L., Hamadat S., Corbalán N. S., González-Lizárraga F., Dos-Santos-Pereira M., Rocca J., et al. (2019). Rifampicin and its derivative rifampicin quinone reduce microglial inflammatory responses and neurodegeneration induced in vitro by α-synuclein fibrillary aggregates. Cells 8:776. 10.3390/cells8080776 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

