Neuropsychiatric Adverse Events During 12 Months of Treatment With Efavirenz in Treatment-Naïve HIV-Infected Patients in China: A Prospective Cohort Study
- PMID: 33716807
- PMCID: PMC7943719
- DOI: 10.3389/fpsyt.2021.579448
Neuropsychiatric Adverse Events During 12 Months of Treatment With Efavirenz in Treatment-Naïve HIV-Infected Patients in China: A Prospective Cohort Study
Abstract
Background: Efavirenz (EFV) is widely used in antiretroviral therapy (ART), but the incidence and risk factors of neuropsychiatric adverse events (NPAEs) after EFV treatment have rarely been studied in Chinese ART naïve patients. Methods: This prospective cohort study assessed HIV-infected patients initiating antiretroviral treatment with EFV to determine prevalence of and factors associated with NPAEs over a 12-month follow-up period using the Hospital Anxiety and Depression Scale (HADS) and the Pittsburgh Sleep Quality Index (PSQI). Results: A total of 546 patients were enrolled. Prevalence of anxiety, depression, and sleep disturbances at baseline were 30.4, 22.7, and 68.1%, respectively. Six patients discontinued treatment due to drug related NPAEs. Treatment was associated with improvements in HADS-A, HADS-D, and PSQI scores over the 12-month follow-up, and the frequencies of patients with anxiety, depression, and sleep disturbances significantly decreased after 12 months. Abnormal baseline HADS-A, HADS-D, and PSQI scores and other factors, including high school education or lower income, unemployment, divorce, and WHO III/IV stages, were associated with severe neuropsychiatric disorders over the 12 months. Conclusions: These findings suggested EFV discontinuation due to NAPEs was low, and the HADS-A, HADS-D, and PSQI scores after 12 months of EFV treatment were associated with several risk factors. The clinicians should keep in mind and routinely screen for the risk factors associated with neuropsychiatric disorders in HIV-infected patients.
Keywords: antiretroviral therapy; anxiety; depression; efavirenz; sleep disturbances.
Copyright © 2021 Hua, Wang, Wang, Shao, Wang, Ye, Su, Jiang, Zhang, Wu, Liu, Li, Mahajan, Li, Sun and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers SS and JC declared a shared affiliation with the authors SM and SW respectively, to the handling editor at the time of review.
Figures
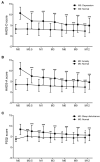
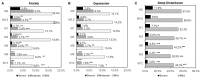
References
-
- WHO Guidelines Approved by the Guidelines Review Committee . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; (2016). - PubMed
-
- Yeni P, Cooper DA, Aboulker JP, Babiker AG, Carey D, Darbyshire JH, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. (2006) 368:287–98. 10.1016/S0140-6736(06)69074-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

