Long-Term Outcomes of the Minimally Invasive Ponto Surgery vs. Linear Incision Technique With Soft Tissue Preservation for Installation of Percutaneous Bone Conduction Devices
- PMID: 33716934
- PMCID: PMC7945693
- DOI: 10.3389/fneur.2021.632987
Long-Term Outcomes of the Minimally Invasive Ponto Surgery vs. Linear Incision Technique With Soft Tissue Preservation for Installation of Percutaneous Bone Conduction Devices
Abstract
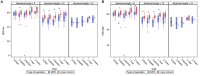
Objective: Comparing the surgical outcomes of the Minimally Invasive Ponto Surgery (MIPS) technique with the linear incision technique with soft tissue preservation (LITT-P) for bone conduction devices after a follow-up of 22 months. Methods: In this multicenter randomized controlled trial, there was the inclusion of 64 adult patients eligible for unilateral surgery. There was 1:1 randomization to the MIPS (test) or the LITT-P (control) group. The primary outcome was an (adverse) soft tissue reaction. Secondary outcomes were pain, loss of sensibility, soft tissue height/overgrowth, skin sagging, implant loss, Implant Stability Quotient measurements, cosmetic scores, and quality of life questionnaires. Results: Sixty-three subjects were analyzed in the intention-to-treat population. No differences were found in the presence of (adverse) soft tissue reactions during complete follow-up. Also, there were no differences in pain, wound dehiscence, skin level, soft tissue overgrowth, and overall quality of life. Loss of sensibility (until 3-month post-surgery), cosmetic scores, and skin sagging outcomes were better in the MIPS group. The Implant Stability Quotient was higher after the LITT-P for different abutment lengths at various points of follow-up. Implant extrusion was nonsignificantly higher after the MIPS (15.2%) compared with LITT-P (3.3%). Conclusion: The long-term results show favorable outcomes for both techniques. The MIPS is a promising technique with some benefits over the LITT-P. Concerns regarding nonsignificantly higher implant loss may be overcome with future developments and research. Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT02438618.
Keywords: MIPS; bone conduction device (BCD); hearing loss; minimally invasive ponto surgery; soft tissue reactions; surgical outcomes; surgical technique; tissue preservation.
Copyright © 2021 Strijbos, Straatman, Calon, Johansson, de Bruijn, van den Berge, Wagenaar, Eichhorn, Janssen, Jonhede, van Tongeren, Holmberg and Stokroos.
Conflict of interest statement
MLJ, SJ, and MH are paid employees of Oticon Medical. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Minimally Invasive Ponto Surgery compared to the linear incision technique without soft tissue reduction for bone conduction hearing implants: study protocol for a randomized controlled trial.Trials. 2016 Nov 9;17(1):540. doi: 10.1186/s13063-016-1662-0. Trials. 2016. PMID: 27829464 Free PMC article. Clinical Trial.
-
Minimally Invasive Ponto Surgery Versus the Linear Incision Technique With Soft Tissue Preservation for Bone Conduction Hearing Implants: A Multicenter Randomized Controlled Trial.Otol Neurotol. 2018 Aug;39(7):882-893. doi: 10.1097/MAO.0000000000001852. Otol Neurotol. 2018. PMID: 29995008 Free PMC article. Clinical Trial.
-
Six-Month Clinical Outcomes for Bone-Anchored Hearing Implants: Comparison Between Minimally Invasive Ponto Surgery and the Linear Incision Technique With Tissue Preservation.Otol Neurotol. 2020 Apr;41(4):e475-e483. doi: 10.1097/MAO.0000000000002562. Otol Neurotol. 2020. PMID: 32176135
-
The efficacy of bone-anchored hearing implant surgery in children: A systematic review.Int J Pediatr Otorhinolaryngol. 2020 May;132:109906. doi: 10.1016/j.ijporl.2020.109906. Epub 2020 Jan 28. Int J Pediatr Otorhinolaryngol. 2020. PMID: 32028192
-
The Ponto bone-anchored hearing system.Adv Otorhinolaryngol. 2011;71:32-40. doi: 10.1159/000323578. Epub 2011 Mar 8. Adv Otorhinolaryngol. 2011. PMID: 21389702 Review.
Cited by
-
Ex vivo Evaluation of a New Drill System for Placement of Percutaneous Bone Conduction Devices.Front Surg. 2022 Mar 21;9:858117. doi: 10.3389/fsurg.2022.858117. eCollection 2022. Front Surg. 2022. PMID: 35388366 Free PMC article.
-
Long-Term Clinical Outcomes for Bone-Anchored Hearing Implants: 3-Year Comparison Between Minimally Invasive Ponto Surgery and the Linear Incision Technique With Tissue Preservation.Otol Neurotol. 2025 Feb 1;46(2):161-169. doi: 10.1097/MAO.0000000000004398. Otol Neurotol. 2025. PMID: 39792980 Free PMC article.
-
Minimally invasive surgery as a new clinical standard for bone anchored hearing implants-real-world data from 10 years of follow-up and 228 surgeries.Front Surg. 2023 Jul 3;10:1209927. doi: 10.3389/fsurg.2023.1209927. eCollection 2023. Front Surg. 2023. PMID: 37465065 Free PMC article.
-
Post-implantation clinical cost analysis between transcutaneous and percutaneous bone conduction devices.Eur Arch Otorhinolaryngol. 2024 Jan;281(1):117-127. doi: 10.1007/s00405-023-08099-2. Epub 2023 Jul 8. Eur Arch Otorhinolaryngol. 2024. PMID: 37421428 Free PMC article.
-
Mechanical and thermal efficiency of a single drill system for bone-anchored hearing implants.PLoS One. 2025 May 30;20(5):e0311026. doi: 10.1371/journal.pone.0311026. eCollection 2025. PLoS One. 2025. PMID: 40445948 Free PMC article.
References
-
- Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. (1981) 2:304–10. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

