Virus-Host Interactions in Foot-and-Mouth Disease Virus Infection
- PMID: 33717061
- PMCID: PMC7952751
- DOI: 10.3389/fimmu.2021.571509
Virus-Host Interactions in Foot-and-Mouth Disease Virus Infection
Abstract
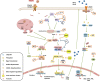
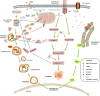
Foot-and-mouth disease (FMD) is a highly contagious disease of cloven-hoofed animals, which has been regarded as a persistent challenge for the livestock industry in many countries. Foot-and-mouth disease virus (FMDV) is the etiological agent of FMD that can spread rapidly by direct and indirect transmission. FMDV is internalized into host cell by the interaction between FMDV capsid proteins and cellular receptors. When the virus invades into the cells, the host antiviral system is quickly activated to suppress the replication of the virus and remove the virus. To retain fitness and host adaptation, various viruses have evolved multiple elegant strategies to manipulate host machine and circumvent the host antiviral responses. Therefore, identification of virus-host interactions is critical for understanding the host defense against virus infections and the pathogenesis of the viral infectious diseases. This review elaborates on the virus-host interactions during FMDV infection to summarize the pathogenic mechanisms of FMD, and we hope it can provide insights for designing effective vaccines or drugs to prevent and control the spread of FMD and other diseases caused by picornaviruses.
Keywords: FMDV; immune dysfunction; interactions; pathogenesis; viral infection.
Copyright © 2021 Li, Wang, Yang, Cao, Zhu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

