The Roles of Immunoregulatory Networks in Severe Drug Hypersensitivity
- PMID: 33717075
- PMCID: PMC7953830
- DOI: 10.3389/fimmu.2021.597761
The Roles of Immunoregulatory Networks in Severe Drug Hypersensitivity
Abstract
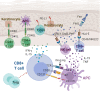
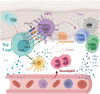
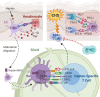
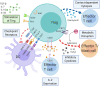
The immunomodulatory effects of regulatory T cells (Tregs) and co-signaling receptors have gained much attention, as they help balance immunogenic and immunotolerant responses that may be disrupted in autoimmune and infectious diseases. Drug hypersensitivity has a myriad of manifestations, which ranges from the mild maculopapular exanthema to the severe Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS). While studies have identified high-risk human leukocyte antigen (HLA) allotypes, the presence of the HLA allotype at risk is not sufficient to elicit drug hypersensitivity. Recent studies have suggested that insufficient regulation by Tregs may play a role in severe hypersensitivity reactions. Furthermore, immune checkpoint inhibitors, such as anti-CTLA-4 or anti-PD-1, in cancer treatment also induce hypersensitivity reactions including SJS/TEN and DRESS/DIHS. Taken together, mechanisms involving both Tregs as well as coinhibitory and costimulatory receptors may be crucial in the pathogenesis of drug hypersensitivity. In this review, we summarize the currently implicated roles of co-signaling receptors and Tregs in delayed-type drug hypersensitivity in the hope of identifying potential pharmacologic targets.
Keywords: Stevens-Johnson Syndrome; contact dermatitis; cosignaling pathways; delayed type hypersensitivity; drug reaction with eosinophilia and systemic symptoms; immune checkpoints; regulatory T cells; toxic epidermal necrolysis.
Copyright © 2021 Hsu, Lu, Fu, Wang, Lu, Lin, Chang, Yeh, Hung, Chung and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Justiz Vaillant AA, Zulfiqar H, Ramphul K. Delayed Hypersensitivity Reactions. Treasure Island (FL: StatPearls; (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

