Validation and Optimization of Host Immunological Bio-Signatures for a Point-of-Care Test for TB Disease
- PMID: 33717089
- PMCID: PMC7952865
- DOI: 10.3389/fimmu.2021.607827
Validation and Optimization of Host Immunological Bio-Signatures for a Point-of-Care Test for TB Disease
Abstract
The development of a non-sputum-based, point-of-care diagnostic test for tuberculosis (TB) is a priority in the global effort to combat this disease, particularly in resource-constrained settings. Previous studies have identified host biomarker signatures which showed potential, but there is a need to validate and refine these for development as a test. We recruited 1,403 adults presenting with symptoms suggestive of pulmonary TB at primary healthcare clinics in six countries from West, East and Southern Africa. Of the study cohort, 326 were diagnosed with TB and 787 with other respiratory diseases, from whom we randomly selected 1005 participants. Using Luminex® technology, we measured the levels of 20 host biomarkers in serum samples which we used to evaluate the diagnostic accuracy of previously identified and novel bio-signatures. Our previously identified seven-marker bio-signature did not perform well (sensitivity: 89%, specificity: 60%). We also identified an optimal, two-marker bio-signature with a sensitivity of 94% and specificity of 69% in patients with no history of previous TB. This signature performed slightly better than C-reactive protein (CRP) alone. The cut-off value for a positive diagnosis differed for human immuno-deficiency virus (HIV)-positive and -negative individuals. Notably, we also found that no signature was able to diagnose TB adequately in patients with a prior history of the disease. We have identified a two-marker, pan-African bio-signature which is more robust than CRP alone and meets the World Health Organization (WHO) target product profile requirements for a triage test in both HIV-negative and HIV-positive individuals. This signature could be incorporated into a point-of-care device, greatly reducing the necessity for expensive confirmatory diagnostics and potentially reducing the number of cases currently lost to follow-up. It might also potentially be useful with individuals unable to provide sputum or with paucibacillary disease. We suggest that the performance of TB diagnostic signatures can be improved by incorporating the HIV-status of the patient. We further suggest that only patients who have never had TB be subjected to a triage test and that those with a history of previous TB be evaluated using more direct diagnostic techniques.
Keywords: M. tuberculosis (M. tb); bio-signature; biomarkers; blood; diagnostic; point of care; validation.
Copyright © 2021 Mutavhatsindi, van der Spuy, Malherbe, Sutherland, Geluk, Mayanja-Kizza, Crampin, Kassa, Howe, Mihret, Sheehama, Nepolo, Günther, Dockrell, Corstjens, Stanley, Walzl, Chegou and the AE-TBC ScreenTB Consortia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



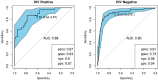

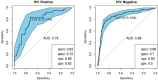


References
-
- World Health Organization . Global tuberculosis report 2018 (2018). WHO. Available at: http://www.who.int/iris/handle/10665/274453 (Accessed April 11, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

