CXCL17 Is a Specific Diagnostic Biomarker for Severe Pandemic Influenza A(H1N1) That Predicts Poor Clinical Outcome
- PMID: 33717172
- PMCID: PMC7953906
- DOI: 10.3389/fimmu.2021.633297
CXCL17 Is a Specific Diagnostic Biomarker for Severe Pandemic Influenza A(H1N1) That Predicts Poor Clinical Outcome
Erratum in
-
Corrigendum: CXCL17 Is a Specific Diagnostic Biomarker for Severe Pandemic Influenza A(H1N1) That Predicts Poor Clinical Outcome.Front Immunol. 2021 May 13;12:700716. doi: 10.3389/fimmu.2021.700716. eCollection 2021. Front Immunol. 2021. PMID: 34054884 Free PMC article.
Abstract
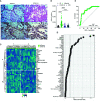
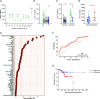
The C-X-C motif chemokine ligand 17 (CXCL17) is chemotactic for myeloid cells, exhibits bactericidal activity, and exerts anti-viral functions. This chemokine is constitutively expressed in the respiratory tract, suggesting a role in lung defenses. However, little is known about the participation of CXCL17 against relevant respiratory pathogens in humans. Here, we evaluated the serum levels and lung tissue expression pattern of CXCL17 in a cohort of patients with severe pandemic influenza A(H1N1) from Mexico City. Peripheral blood samples obtained on admission and seven days after hospitalization were processed for determinations of serum CXCL17 levels by enzyme-linked immunosorbent assay (ELISA). The expression of CXCL17 was assessed by immunohistochemistry (IHQ) in lung autopsy specimens from patients that succumbed to the disease. Serum CXCL17 levels were also analyzed in two additional comparative cohorts of coronavirus disease 2019 (COVID-19) and pulmonary tuberculosis (TB) patients. Additionally, the expression of CXCL17 was tested in lung autopsy specimens from COVID-19 patients. A total of 122 patients were enrolled in the study, from which 68 had pandemic influenza A(H1N1), 24 had COVID-19, and 30 with PTB. CXCL17 was detected in post-mortem lung specimens from patients that died of pandemic influenza A(H1N1) and COVID-19. Interestingly, serum levels of CXCL17 were increased only in patients with pandemic influenza A(H1N1), but not COVID-19 and PTB. CXCL17 not only differentiated pandemic influenza A(H1N1) from other respiratory infections but showed prognostic value for influenza-associated mortality and renal failure in machine-learning algorithms and regression analyses. Using cell culture assays, we also identified that human alveolar A549 cells and peripheral blood monocyte-derived macrophages increase their CXCL17 production capacity after influenza A(H1N1) pdm09 virus infection. Our results for the first time demonstrate an induction of CXCL17 specifically during pandemic influenza A(H1N1), but not COVID-19 and PTB in humans. These findings could be of great utility to differentiate influenza and COVID-19 and to predict poor prognosis specially at settings of high incidence of pandemic A(H1N1). Future studies on the role of CXCL17 not only in severe pandemic influenza, but also in seasonal influenza, COVID-19, and PTB are required to validate our results.
Keywords: COVID-19; CXCL17; SARS-CoV-2; chemokines; influenza A(H1N1); tuberculosis.
Copyright © 2021 Choreño-Parra, Jiménez-Álvarez, Ramírez-Martínez, Sandoval-Vega, Salinas-Lara, Sánchez-Garibay, Luna-Rivero, Hernández-Montiel, Fernández-López, Cabrera-Cornejo, Choreño-Parra, Cruz-Lagunas, Domínguez, Márquez-García, Cabello-Gutiérrez, Bolaños-Morales, Mena-Hernández, Delgado-Zaldivar, Rebolledo-García, Guadarrama-Ortiz, Regino-Zamarripa, Mendoza-Milla, García-Latorre, Rodiguez-Reyna, Cervántes-Rosete, Hernández-Cárdenas, Khader, Zlotnik and Zúñiga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Expression of Surfactant Protein D Distinguishes Severe Pandemic Influenza A(H1N1) from Coronavirus Disease 2019.J Infect Dis. 2021 Jul 2;224(1):21-30. doi: 10.1093/infdis/jiab113. J Infect Dis. 2021. PMID: 33668070 Free PMC article.
-
CXCL17 Is Dispensable during Hypervirulent Mycobacterium tuberculosis HN878 Infection in Mice.Immunohorizons. 2021 Sep 24;5(9):752-759. doi: 10.4049/immunohorizons.2100048. Immunohorizons. 2021. PMID: 34561226 Free PMC article.
-
Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols.Elife. 2021 Apr 16;10:e65774. doi: 10.7554/eLife.65774. Elife. 2021. PMID: 33861198 Free PMC article.
-
Comparison of patients hospitalized with COVID-19, H7N9 and H1N1.Infect Dis Poverty. 2020 Dec 2;9(1):163. doi: 10.1186/s40249-020-00781-5. Infect Dis Poverty. 2020. PMID: 33261654 Free PMC article.
-
Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review.Chest. 2021 Jan;159(1):73-84. doi: 10.1016/j.chest.2020.09.259. Epub 2020 Oct 7. Chest. 2021. PMID: 33038391 Free PMC article.
Cited by
-
CXCL17 is a proinflammatory chemokine and promotes neutrophil trafficking.J Leukoc Biol. 2024 May 29;115(6):1177-1182. doi: 10.1093/jleuko/qiae028. J Leukoc Biol. 2024. PMID: 38298146 Free PMC article.
-
The cryptic role of CXCL17/CXCR8 axis in the pathogenesis of cancers: a review of the latest evidence.J Cell Commun Signal. 2023 Sep;17(3):409-422. doi: 10.1007/s12079-022-00699-7. Epub 2022 Nov 9. J Cell Commun Signal. 2023. PMID: 36352331 Free PMC article. Review.
-
Clinical and Immunological Factors That Distinguish COVID-19 From Pandemic Influenza A(H1N1).Front Immunol. 2021 Apr 21;12:593595. doi: 10.3389/fimmu.2021.593595. eCollection 2021. Front Immunol. 2021. PMID: 33995342 Free PMC article. Clinical Trial.
-
Comparing the Cytokine Storms of COVID-19 and Pandemic Influenza.J Interferon Cytokine Res. 2022 Aug;42(8):369-392. doi: 10.1089/jir.2022.0029. Epub 2022 Jun 7. J Interferon Cytokine Res. 2022. PMID: 35674675 Free PMC article.
-
The Chemokine System as a Key Regulator of Pulmonary Fibrosis: Converging Pathways in Human Idiopathic Pulmonary Fibrosis (IPF) and the Bleomycin-Induced Lung Fibrosis Model in Mice.Cells. 2024 Dec 12;13(24):2058. doi: 10.3390/cells13242058. Cells. 2024. PMID: 39768150 Free PMC article. Review.
References
-
- Srivastava R, Hernandez-Ruiz M, Khan AA, Fouladi MA, Kim GJ, Ly VT, et al. CXCL17 Chemokine-Dependent Mobilization of CXCR8(+)CD8(+) Effector Memory and Tissue-Resident Memory T Cells in the Vaginal Mucosa Is Associated with Protection against Genital Herpes. J Immunol (2018) 200(8):2915–26. 10.4049/jimmunol.1701474 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

