Standardization and Quality Assessment Under the Perspective of Automated Computer-Assisted HEp-2 Immunofluorescence Assay Systems
- PMID: 33717188
- PMCID: PMC7947926
- DOI: 10.3389/fimmu.2021.638863
Standardization and Quality Assessment Under the Perspective of Automated Computer-Assisted HEp-2 Immunofluorescence Assay Systems
Abstract
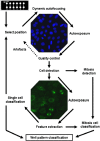
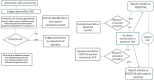
The recent availability of automated computer-assisted diagnosis (CAD) systems for the reading and interpretation of the anti-nuclear antibody (ANA) test performed with the indirect immunofluorescence (IIF) method on HEp-2 cells, has improved the reproducibility of the results and initiated a process of harmonization of this test. Furthermore, CAD systems provide quantitative expression of fluorescence intensity, allowing the introduction of objective quality control procedures to the monitoring of the entire process. The calibration of the reading systems and the automated image interpretation are essential prerequisites for obtaining reproducible and harmonized IIF test results and form the basis for standardization, regardless of the computer algorithms used in the different systems. The use of automated CAD systems, facilitating control procedures, represents a step forward for the quality certification of the laboratory.
Keywords: anti-nuclear antibodies; automation; computer-assisted systems; harmonization; immunofluorescence; standardization.
Copyright © 2021 Cinquanta, Bizzaro and Pesce.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The burden of the variability introduced by the HEp-2 assay kit and the CAD system in ANA indirect immunofluorescence test.Immunol Res. 2017 Feb;65(1):345-354. doi: 10.1007/s12026-016-8845-3. Immunol Res. 2017. PMID: 27456204
-
Automated Processing and Evaluation of Anti-Nuclear Antibody Indirect Immunofluorescence Testing.Front Immunol. 2018 May 4;9:927. doi: 10.3389/fimmu.2018.00927. eCollection 2018. Front Immunol. 2018. PMID: 29780386 Free PMC article.
-
Automated tests of ANA immunofluorescence as throughput autoantibody detection technology: strengths and limitations.BMC Med. 2014 Mar 3;12:38. doi: 10.1186/1741-7015-12-38. BMC Med. 2014. PMID: 24589329 Free PMC article. Review.
-
A comparative study on the reliability of an automated system for the evaluation of cell-based indirect immunofluorescence.Autoimmun Rev. 2012 Aug;11(10):713-6. doi: 10.1016/j.autrev.2011.12.010. Epub 2012 Jan 16. Autoimmun Rev. 2012. PMID: 22269861 Review.
-
EUROPattern Suite technology for computer-aided immunofluorescence microscopy in autoantibody diagnostics.Lupus. 2015 Apr;24(4-5):516-29. doi: 10.1177/0961203314559635. Lupus. 2015. PMID: 25801895 Review.
Cited by
-
Recognition of rare antinuclear antibody patterns based on a novel attention-based enhancement framework.Brief Bioinform. 2024 Jan 22;25(2):bbad531. doi: 10.1093/bib/bbad531. Brief Bioinform. 2024. PMID: 38279651 Free PMC article.
-
Strong Association of the Myriad Discrete Speckled Nuclear Pattern With Anti-SS-A/Ro60 Antibodies: Consensus Experience of Four International Expert Centers.Front Immunol. 2021 Oct 5;12:730102. doi: 10.3389/fimmu.2021.730102. eCollection 2021. Front Immunol. 2021. PMID: 34675922 Free PMC article.
-
Comparison of the Capacity of Several Machine Learning Tools to Assist Immunofluorescence-Based Detection of Anti-Neutrophil Cytoplasmic Antibodies.Int J Mol Sci. 2024 Mar 13;25(6):3270. doi: 10.3390/ijms25063270. Int J Mol Sci. 2024. PMID: 38542244 Free PMC article.
-
Rational development and application of biomarkers in the field of autoimmunity: A conceptual framework guiding clinicians and researchers.J Transl Autoimmun. 2022 Mar 6;5:100151. doi: 10.1016/j.jtauto.2022.100151. eCollection 2022. J Transl Autoimmun. 2022. PMID: 35309737 Free PMC article. Review.
References
-
- American College of Rheumatology . Current practice issues: ACR tracking concerns about ANA testing results. Atlanta, GA: American College of Rheumatology; (2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

