Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies
- PMID: 33717193
- PMCID: PMC7943455
- DOI: 10.3389/fimmu.2021.640093
Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies
Abstract
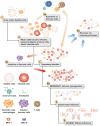
COVID-19 (SARS-CoV-2) disease severity and stages varies from asymptomatic, mild flu-like symptoms, moderate, severe, critical, and chronic disease. COVID-19 disease progression include lymphopenia, elevated proinflammatory cytokines and chemokines, accumulation of macrophages and neutrophils in lungs, immune dysregulation, cytokine storms, acute respiratory distress syndrome (ARDS), etc. Development of vaccines to severe acute respiratory syndrome (SARS), Middle East Respiratory Syndrome coronavirus (MERS-CoV), and other coronavirus has been difficult to create due to vaccine induced enhanced disease responses in animal models. Multiple betacoronaviruses including SARS-CoV-2 and SARS-CoV-1 expand cellular tropism by infecting some phagocytic cells (immature macrophages and dendritic cells) via antibody bound Fc receptor uptake of virus. Antibody-dependent enhancement (ADE) may be involved in the clinical observation of increased severity of symptoms associated with early high levels of SARS-CoV-2 antibodies in patients. Infants with multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 may also have ADE caused by maternally acquired SARS-CoV-2 antibodies bound to mast cells. ADE risks associated with SARS-CoV-2 has implications for COVID-19 and MIS-C treatments, B-cell vaccines, SARS-CoV-2 antibody therapy, and convalescent plasma therapy for patients. SARS-CoV-2 antibodies bound to mast cells may be involved in MIS-C and multisystem inflammatory syndrome in adults (MIS-A) following initial COVID-19 infection. SARS-CoV-2 antibodies bound to Fc receptors on macrophages and mast cells may represent two different mechanisms for ADE in patients. These two different ADE risks have possible implications for SARS-CoV-2 B-cell vaccines for subsets of populations based on age, cross-reactive antibodies, variabilities in antibody levels over time, and pregnancy. These models place increased emphasis on the importance of developing safe SARS-CoV-2 T cell vaccines that are not dependent upon antibodies.
Keywords: ADE; COVID-19; MIS-C; SARS-CoV-2; antibody dependent enhancement; multisystem inflammatory syndrome.
Copyright © 2021 Ricke.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE. Available online at: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594... (accessed January 28, 2021).
-
- Yan X, Hao Q, Mu Y, Timani KA, Ye L, Zhu Y, et al. . Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. Int J Biochem Cell Biol. (2006) 38:1417–28. 10.1016/j.biocel.2006.02.003 - DOI - PMC - PubMed
-
- Tomera K, Malone R, Kittah J. Hospitalized COVID-19 patients treated with celecoxib and high dose famotidine adjuvant therapy show significant clinical responses. SSRN [Preprint]. (2020) 10.2139/ssrn.3646583 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

