Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine
- PMID: 33717194
- PMCID: PMC7943459
- DOI: 10.3389/fimmu.2021.640190
Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine
Abstract
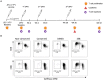
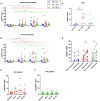
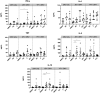
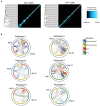
The epidemic spread of Zika virus (ZIKV), associated with devastating neurologic syndromes, has driven the development of multiple ZIKV vaccines candidates. An effective vaccine should induce ZIKV-specific T cell responses, which are shown to improve the establishment of humoral immunity and contribute to viral clearance. Here we investigated how previous immunization against Japanese encephalitis virus (JEV) and yellow fever virus (YFV) influences T cell responses elicited by a Zika purified-inactivated virus (ZPIV) vaccine. We demonstrate that three doses of ZPIV vaccine elicited robust CD4 T cell responses to ZIKV structural proteins, while ZIKV-specific CD4 T cells in pre-immunized individuals with JEV vaccine, but not YFV vaccine, were more durable and directed predominantly toward conserved epitopes, which elicited Th1 and Th2 cytokine production. In addition, T cell receptor repertoire analysis revealed preferential expansion of cross-reactive clonotypes between JEV and ZIKV, suggesting that pre-existing immunity against JEV may prime the establishment of stronger CD4 T cell responses to ZPIV vaccination. These CD4 T cell responses correlated with titers of ZIKV-neutralizing antibodies in the JEV pre-vaccinated group, but not in flavivirus-naïve or YFV pre-vaccinated individuals, suggesting a stronger contribution of CD4 T cells in the generation of neutralizing antibodies in the context of JEV-ZIKV cross-reactivity.
Keywords: CD4 T cell; TCR repertoire; cross-reactivity; flavivirus; vaccine; zika virus.
Copyright © 2021 Lima, Moon, Darko, De La Barrera, Lin, Koren, Jarman, Eckels, Thomas, Michael, Modjarrad, Douek and Trautmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JB declared a past co-authorship with the following authors NM and KM, to the handling editor at the time of review.
Figures


Similar articles
-
Priming with Japanese encephalitis virus or yellow fever virus vaccination led to the recognition of multiple flaviviruses without boosting antibody responses induced by an inactivated Zika virus vaccine.EBioMedicine. 2023 Nov;97:104815. doi: 10.1016/j.ebiom.2023.104815. Epub 2023 Oct 2. EBioMedicine. 2023. PMID: 37793212 Free PMC article.
-
T Cell Responses Induced by Attenuated Flavivirus Vaccination Are Specific and Show Limited Cross-Reactivity with Other Flavivirus Species.J Virol. 2020 May 4;94(10):e00089-20. doi: 10.1128/JVI.00089-20. Print 2020 May 4. J Virol. 2020. PMID: 32132233 Free PMC article.
-
Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in adults primed with a Japanese encephalitis virus or yellow fever virus vaccine in the USA: a phase 1, randomised, double-blind, placebo-controlled clinical trial.Lancet Infect Dis. 2023 Oct;23(10):1175-1185. doi: 10.1016/S1473-3099(23)00192-5. Epub 2023 Jun 27. Lancet Infect Dis. 2023. PMID: 37390836 Free PMC article. Clinical Trial.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Immune Responses to Dengue and Zika Viruses-Guidance for T Cell Vaccine Development.Int J Environ Res Public Health. 2018 Feb 23;15(2):385. doi: 10.3390/ijerph15020385. Int J Environ Res Public Health. 2018. PMID: 29473899 Free PMC article. Review.
Cited by
-
Assessing the effect of beta-propiolactone inactivation on the antigenicity and immunogenicity of cluster 2.1 duck Tembusu virus.Poult Sci. 2025 Mar;104(3):104878. doi: 10.1016/j.psj.2025.104878. Epub 2025 Feb 2. Poult Sci. 2025. PMID: 39919563 Free PMC article.
-
Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases.Viruses. 2022 Jun 2;14(6):1213. doi: 10.3390/v14061213. Viruses. 2022. PMID: 35746683 Free PMC article. Review.
-
Japanese Encephalitis Vaccine Generates Cross-Reactive Memory T Cell Responses to Zika Virus in Humans.J Trop Med. 2022 Nov 19;2022:8379286. doi: 10.1155/2022/8379286. eCollection 2022. J Trop Med. 2022. PMID: 36444358 Free PMC article.
-
Antigen-specific T cell responses following single and co-administration of tick-borne encephalitis, Japanese encephalitis, and yellow fever virus vaccines: Results from an open-label, non-randomized clinical trial-cohort.PLoS Negl Trop Dis. 2025 Feb 28;19(2):e0012693. doi: 10.1371/journal.pntd.0012693. eCollection 2025 Feb. PLoS Negl Trop Dis. 2025. PMID: 40019865 Free PMC article. Clinical Trial.
-
Antibodies from dengue patients with prior exposure to Japanese encephalitis virus are broadly neutralizing against Zika virus.Commun Biol. 2024 Jan 24;7(1):15. doi: 10.1038/s42003-023-05661-w. Commun Biol. 2024. PMID: 38267569 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

