Crosstalk Between Trophoblasts and Decidual Immune Cells: The Cornerstone of Maternal-Fetal Immunotolerance
- PMID: 33717198
- PMCID: PMC7947923
- DOI: 10.3389/fimmu.2021.642392
Crosstalk Between Trophoblasts and Decidual Immune Cells: The Cornerstone of Maternal-Fetal Immunotolerance
Abstract
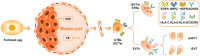
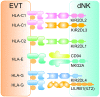
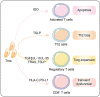
The success of pregnancy relies on the fine adjustment of the maternal immune system to tolerate the allogeneic fetus. Trophoblasts carrying paternal antigens are the only fetal-derived cells that come into direct contact with the maternal immune cells at the maternal-fetal interface. The crosstalk between trophoblasts and decidual immune cells (DICs) via cell-cell direct interaction and soluble factors such as chemokines and cytokines is a core event contributing to the unique immunotolerant microenvironment. Abnormal trophoblasts-DICs crosstalk can lead to dysregulated immune situations, which is well known to be a potential cause of a series of pregnancy complications including recurrent spontaneous abortion (RSA), which is the most common one. Immunotherapy has been applied to RSA. However, its development has been far less rapid or mature than that of cancer immunotherapy. Elucidating the mechanism of maternal-fetal immune tolerance, the theoretical basis for RSA immunotherapy, not only helps to understand the establishment and maintenance of normal pregnancy but also provides new therapeutic strategies and promotes the progress of immunotherapy against pregnancy-related diseases caused by disrupted immunotolerance. In this review, we focus on recent progress in the maternal-fetal immune tolerance mediated by trophoblasts-DICs crosstalk and clinical application of immunotherapy in RSA. Advancement in this area will further accelerate the basic research and clinical transformation of reproductive immunity and tumor immunity.
Keywords: decidual immune cells; immunotherapy; maternal-fetal immunotolerance; recurrent spontaneous abortion; trophoblasts.
Copyright © 2021 Xu, Li, Sang, Li and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

