Hypocrisy Around Medical Patient Data: Issues of Access for Biomedical Research, Data Quality, Usefulness for the Purpose and Omics Data as Game Changer
- PMID: 33717311
- PMCID: PMC7747340
- DOI: 10.1007/s41649-019-00085-3
Hypocrisy Around Medical Patient Data: Issues of Access for Biomedical Research, Data Quality, Usefulness for the Purpose and Omics Data as Game Changer
Abstract
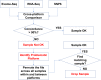
Whether due to simplicity or hypocrisy, the question of access to patient data for biomedical research is widely seen in the public discourse only from the angle of patient privacy. At the same time, the desire to live and to live without disability is of much higher value to the patients. This goal can only be achieved by extracting research insight from patient data in addition to working on model organisms, something that is well understood by many patients. Yet, most biomedical researchers working outside of clinics and hospitals are denied access to patient records when, at the same time, clinicians who guard the patient data are not optimally prepared for the data's analysis. Medical data collection is a time- and cost-intensive process that is most of all tedious, with few elements of intellectual and emotional satisfaction on its own. In this process, clinicians and bioinformaticians, each group with their own interests, have to join forces with the goal to generate medical data sets both from clinical trials and from routinely collected electronic health records that are, as much as possible, free from errors and obvious inconsistencies. The data cleansing effort as we have learned during curation of Singaporean clinical trial data is not a trivial task. The introduction of omics and sophisticated imaging modalities into clinical practice that are only partially interpreted in terms of diagnosis and therapy with today's level of knowledge warrant the creation of clinical databases with full patient history. This opens up opportunities for re-analyses and cross-trial studies at future time points with more sophisticated analyses of the same data, the collection of which is very expensive.
Keywords: Clinical patient data quality; Electronic health record; Genome sequencing; Omics data; Patient data privacy.
© The Author(s) 2019.
Figures
Similar articles
-
The project data sphere initiative: accelerating cancer research by sharing data.Oncologist. 2015 May;20(5):464-e20. doi: 10.1634/theoncologist.2014-0431. Epub 2015 Apr 15. Oncologist. 2015. PMID: 25876994 Free PMC article.
-
The effectiveness of internet-based e-learning on clinician behavior and patient outcomes: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Jan;13(1):52-64. doi: 10.11124/jbisrir-2015-1919. JBI Database System Rev Implement Rep. 2015. PMID: 26447007
-
Beware the Medical-Industrial Complex.Oncologist. 1996;1(4):IV-V. Oncologist. 1996. PMID: 10388005
-
Key concepts to assess the readiness of data for international research: data quality, lineage and provenance, extraction and processing errors, traceability, and curation. Contribution of the IMIA Primary Health Care Informatics Working Group.Yearb Med Inform. 2011;6:112-20. Yearb Med Inform. 2011. PMID: 21938335 Review.
-
A review of the impact of utilising electronic medical records for clinical research recruitment.Clin Trials. 2019 Apr;16(2):194-203. doi: 10.1177/1740774519829709. Epub 2019 Feb 15. Clin Trials. 2019. PMID: 30764659 Review.
Cited by
-
Digital Health Data Quality Issues: Systematic Review.J Med Internet Res. 2023 Mar 31;25:e42615. doi: 10.2196/42615. J Med Internet Res. 2023. PMID: 37000497 Free PMC article.
-
The ethical aspects of exposome research: a systematic review.Exposome. 2023 Apr 12;3(1):osad004. doi: 10.1093/exposome/osad004. Exposome. 2023. PMID: 37745046 Free PMC article.
-
A guide to sharing open healthcare data under the General Data Protection Regulation.Sci Data. 2023 Jun 24;10(1):404. doi: 10.1038/s41597-023-02256-2. Sci Data. 2023. PMID: 37355751 Free PMC article.
-
Ethics in the Era of Big Data.Asian Bioeth Rev. 2019 Jun 18;11(2):169-171. doi: 10.1007/s41649-019-00092-4. eCollection 2019 Jun. Asian Bioeth Rev. 2019. PMID: 33717310 Free PMC article. No abstract available.
References
-
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Research. 2013;41(D1):D991–D995. doi: 10.1093/nar/gks1193. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
