The efficacy and safety of the combination of axitinib and pembrolizumab-activated autologous DC-CIK cell immunotherapy for patients with advanced renal cell carcinoma: a phase 2 study
- PMID: 33717483
- PMCID: PMC7927618
- DOI: 10.1002/cti2.1257
The efficacy and safety of the combination of axitinib and pembrolizumab-activated autologous DC-CIK cell immunotherapy for patients with advanced renal cell carcinoma: a phase 2 study
Abstract
Objectives: Although axitinib has achieved a preferable response rate for advanced renal cell carcinoma (RCC), patient survival remains unsatisfactory. In this study, we evaluated the efficacy and safety of a combination treatment of axitinib and a low dose of pembrolizumab-activated autologous dendritic cells-co-cultured cytokine-induced killer cells in patients with advanced RCC.
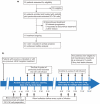
Methods: All adult patients, including treatment-naive or pretreated with VEGF-targeted agents, were enrolled from May 2016 to March 2019. Patients received axitinib 5 mg twice daily and pembrolizumab-activated dendritic cells-co-cultured cytokine-induced killer cells intravenously weekly for the first four cycles, every 2 weeks for the next four cycles, and every month thereafter.
Results: The 43 patients (22 untreated and 21 previously treated) showed a median progression-free survival (mPFS) of 14.7 months (95% CI, 11.16-18.30). mPFS in treatment-naive patients was 18.2 months, as compared with 14.4 months in pretreated patients (log-rank P-value = 0.07). Overall response rates were 25.6% (95% CI, 13.5-41.2%). Grade 3 or higher adverse events occurred in 5% of patients included hypertension (11.6%) and palmar-plantar erythrodysesthesia (7.0%). Peripheral blood lymphocyte immunophenotype and serum cytokine profile analyses demonstrated increased antitumor immunity after combination treatment particularly in patients with a long-term survival benefit, while those with a minimal survival benefit demonstrated an elevated proportion of peripheral CD8+TIM3+ T cells and lower serum-level immunostimulatory cytokine profile.
Conclusions: The combination therapy was active and well tolerated for treatment of advanced RCC, either as first- or second-line treatment following other targeted agents. Changes in immunophenotype and serum cytokine profile may be used as prognostic biomarkers.
Keywords: advanced renal cell carcinoma; axitinib; dendritic cells and cytokine‐induced killer cells immunotherapy; pembrolizumab.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial.Lancet Oncol. 2020 Dec;21(12):1563-1573. doi: 10.1016/S1470-2045(20)30436-8. Epub 2020 Oct 23. Lancet Oncol. 2020. PMID: 33284113 Clinical Trial.
-
Axitinib plus pembrolizumab in patients with advanced renal-cell carcinoma: Long-term efficacy and safety from a phase Ib trial.Eur J Cancer. 2021 Mar;145:1-10. doi: 10.1016/j.ejca.2020.12.009. Epub 2021 Jan 4. Eur J Cancer. 2021. PMID: 33412465 Clinical Trial.
-
Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial.Lancet Oncol. 2018 Apr;19(4):451-460. doi: 10.1016/S1470-2045(18)30107-4. Epub 2018 Mar 9. Lancet Oncol. 2018. PMID: 29530667 Clinical Trial.
-
The European Medicines Agency approval of axitinib (Inlyta) for the treatment of advanced renal cell carcinoma after failure of prior treatment with sunitinib or a cytokine: summary of the scientific assessment of the committee for medicinal products for human use.Oncologist. 2015 Feb;20(2):196-201. doi: 10.1634/theoncologist.2014-0177. Epub 2015 Jan 23. Oncologist. 2015. PMID: 25616431 Free PMC article. Review.
-
Avelumab and axitinib in the treatment of renal cell carcinoma: safety and efficacy.Expert Rev Anticancer Ther. 2020 May;20(5):343-354. doi: 10.1080/14737140.2020.1756780. Epub 2020 May 7. Expert Rev Anticancer Ther. 2020. PMID: 32293937 Review.
Cited by
-
Current Treatment Options for Renal Cell Carcinoma: Focus on Cell-Based Immunotherapy.Cancers (Basel). 2024 Mar 19;16(6):1209. doi: 10.3390/cancers16061209. Cancers (Basel). 2024. PMID: 38539542 Free PMC article. Review.
-
XELOX (capecitabine plus oxaliplatin) plus bevacizumab (anti-VEGF-A antibody) with or without adoptive cell immunotherapy in the treatment of patients with previously untreated metastatic colorectal cancer: a multicenter, open-label, randomized, controlled, phase 3 trial.Signal Transduct Target Ther. 2024 Apr 3;9(1):79. doi: 10.1038/s41392-024-01788-2. Signal Transduct Target Ther. 2024. PMID: 38565886 Free PMC article. Clinical Trial.
-
Clinical value of combining epirubicin with mindfulness intervention in patients with urinary system tumors and depression.World J Psychiatry. 2025 Jan 19;15(1):98737. doi: 10.5498/wjp.v15.i1.98737. eCollection 2025 Jan 19. World J Psychiatry. 2025. PMID: 39831015 Free PMC article.
-
Case report: Dendritic cell-cytokine induced killer cell therapy in subjects with chronic lymphocytic leukemia and peritoneal cancer.Front Med (Lausanne). 2023 Oct 9;10:1240330. doi: 10.3389/fmed.2023.1240330. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37877016 Free PMC article.
References
-
- Ferlay J, Colombet M, Soerjomataram I et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941–1953. - PubMed
-
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008; 34: 193–205. - PubMed
-
- Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol 2009; 27: 3225–3234. - PubMed
-
- McDermott DF, Regan MM, Clark JI et al. Randomized phase III trial of high‐dose interleukin‐2 versus subcutaneous interleukin‐2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005; 23: 133–141. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
