A population-based predictive model predicting candidate for primary tumor surgery in patients with metastatic esophageal cancer
- PMID: 33717560
- PMCID: PMC7947545
- DOI: 10.21037/jtd-20-2347
A population-based predictive model predicting candidate for primary tumor surgery in patients with metastatic esophageal cancer
Abstract
Background: The survival benefit of primary tumor surgery for metastatic esophageal cancer (mEC) patients has been observed, but methods for discriminating which individual patients would benefit from surgery have been poorly defined. Herein, a predictive model was developed to test the hypothesis that only certain metastatic patients would gain a survival benefit from primary tumor surgery.
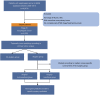
Methods: Clinical data for patients with mEC were extracted from the Surveillance, Epidemiology and End Results (SEER) database [2004-2016] and then divided into surgery and no-surgery groups according to whether surgery was performed on the primary tumor. Propensity-score-matching (PSM) was performed to balance the confounding factors. We hypothesized that the patients who had undergone surgery and lived longer than the median cancer-specific-survival (CSS) of the no-surgery group could benefit from surgery. We constructed a nomogram to predict surgery benefit potential based on multivariable logistic-regression analysis using preoperative factors. The predictive performance of the nomogram was evaluated by the area under the receiver operating characteristic (AUC) and calibration curves. The clinical application value of the nomogram was estimated with decision curve analysis (DCA).
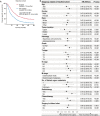
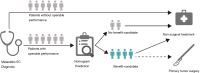
Results: A total of 5,250 eligible patients with mEC were identified, and 9.4% [492] received primary tumor surgery. After PSM, CSS for the surgery group was significantly longer [median: 19 vs. 9 months; hazard ratio (HR) 0.52, P<0.001] compared with the no-surgery group. Among the surgery group, 69.3% [327] survived >9 months (surgery-beneficial group). The prediction nomogram showed good discrimination both in training and validation sets (AUC: 0.72 and 0.70, respectively), and the calibration curves indicated a good consistency. DCA demonstrated that the nomogram was clinically useful. According to this nomogram, surgery patients were classified into two groups: no-benefit-candidate and benefit-candidate. The benefit-candidate group was associated with longer survival than the no-benefit-candidate group (median CSS: 19 vs. 6.5 months, P<0.001). Additionally, there was no difference in survival between the no-benefit-candidate and no-surgery groups (median CSS: 6.5 vs. 9 months, P=0.070).
Conclusions: A predictive model was created for the selection of candidates for surgical treatment among mEC patients. This predictive model might be used to select patients who may benefit from primary tumor surgery.
Keywords: Esophageal cancer (EC); metastatic; predictive model; surgery.
2021 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2347). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
A Model for Identifying Optimal Patients for Primary Tumor Resection in Patients With Metastatic Bladder Cancer.Front Oncol. 2022 Jan 19;11:809664. doi: 10.3389/fonc.2021.809664. eCollection 2021. Front Oncol. 2022. PMID: 35127521 Free PMC article.
-
Selection of Optimal Candidates for Cytoreductive Nephrectomy in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Predictive Model Based on SEER Database.Front Oncol. 2022 Jan 21;12:814512. doi: 10.3389/fonc.2022.814512. eCollection 2022. Front Oncol. 2022. PMID: 35127544 Free PMC article.
-
Distinguishing optimal candidates for primary tumor resection in patients with metastatic lung adenocarcinoma: A predictive model based on propensity score matching.Heliyon. 2024 Mar 26;10(7):e27768. doi: 10.1016/j.heliyon.2024.e27768. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38690000 Free PMC article.
-
A visualized model for identifying optimal candidates for aggressive locoregional surgical treatment in patients with bone metastases from breast cancer.Front Endocrinol (Lausanne). 2023 Oct 5;14:1266679. doi: 10.3389/fendo.2023.1266679. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37867528 Free PMC article.
-
A Novel Nomogram Model to Identify Candidates and Predict the Possibility of Benefit From Primary Tumor Resection Among Female Patients With Metastatic Infiltrating Duct Carcinoma of the Breast: A Large Cohort Study.Front Oncol. 2022 Feb 14;12:798016. doi: 10.3389/fonc.2022.798016. eCollection 2022. Front Oncol. 2022. PMID: 35237513 Free PMC article.
Cited by
-
A Model for Identifying Optimal Patients for Primary Tumor Resection in Patients With Metastatic Bladder Cancer.Front Oncol. 2022 Jan 19;11:809664. doi: 10.3389/fonc.2021.809664. eCollection 2021. Front Oncol. 2022. PMID: 35127521 Free PMC article.
-
Short-term response might influence the treatment-related benefit of adjuvant chemotherapy after concurrent chemoradiotherapy for esophageal squamous cell carcinoma patients.Radiat Oncol. 2021 Oct 2;16(1):195. doi: 10.1186/s13014-021-01921-3. Radiat Oncol. 2021. PMID: 34600574 Free PMC article.
-
Models for Predicting Early Death in Patients With Stage IV Esophageal Cancer: A Surveillance, Epidemiology, and End Results-Based Cohort Study.Cancer Control. 2022 Jan-Dec;29:10732748211072976. doi: 10.1177/10732748211072976. Cancer Control. 2022. PMID: 35037487 Free PMC article.
-
A machine learning model predicting candidates for surgical treatment modality in patients with distant metastatic esophageal adenocarcinoma: A propensity score-matched analysis.Front Oncol. 2022 Jul 22;12:862536. doi: 10.3389/fonc.2022.862536. eCollection 2022. Front Oncol. 2022. PMID: 35936753 Free PMC article.
-
The Effect of the Appropriate Timing of Radiotherapy on Survival Benefit in Patients with Metastatic Esophageal Cancer Who Have Undergone Resection of Primary Site: A SEER Database Analysis.J Oncol. 2022 Mar 16;2022:6086953. doi: 10.1155/2022/6086953. eCollection 2022. J Oncol. 2022. PMID: 35342414 Free PMC article.
References
-
- Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol 1999;26:12-20. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
