Chiral Systems Made from DNA
- PMID: 33717850
- PMCID: PMC7927625
- DOI: 10.1002/advs.202003113
Chiral Systems Made from DNA
Abstract
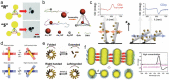
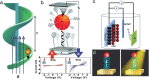
The very chemical structure of DNA that enables biological heredity and evolution has non-trivial implications for the self-organization of DNA molecules into larger assemblies and provides limitless opportunities for building functional nanostructures. This progress report discusses the natural organization of DNA into chiral structures and recent advances in creating synthetic chiral systems using DNA as a building material. How nucleic acid chirality naturally comes into play in a diverse array of situations is considered first, at length scales ranging from an individual nucleotide to entire chromosomes. Thereafter, chiral liquid crystal phases formed by dense DNA mixtures are discussed, including the ongoing efforts to understand their origins. The report then summarizes recent efforts directed toward building chiral structures, and other structures of complex topology, using the principle of DNA self-assembly. Discussed last are existing and proposed functional man-made nanostructures designed to either probe or harness DNA's chirality, from plasmonics and spintronics to biosensing.
Keywords: DNA origami; liquid crystals; nanotechnology; plasmonics; self‐assembly.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cozzarelli N. R., Science 1980, 207, 953. - PubMed
-
- Ivanov V., Minchenkova L., Schyolkina A., Poletayev A., Biopolymers 1973, 12, 89. - PubMed
-
- Zimmerman S. B., Pheiffer B. H., J. Mol. Biol. 1979, 135, 1023. - PubMed
-
- Leslie A., Arnott S., Chandrasekaran R., Ratliff R., J. Mol. Biol. 1980, 143, 49. - PubMed
-
- Pilet J., Brahms J., Nat. New Biol. 1972, 236, 99. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
