Metal-Organic Frameworks for Liquid Phase Applications
- PMID: 33717851
- PMCID: PMC7927635
- DOI: 10.1002/advs.202003143
Metal-Organic Frameworks for Liquid Phase Applications
Abstract
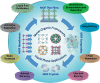
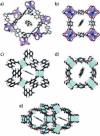
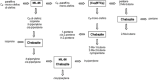
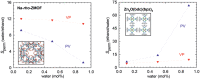
In the last two decades, metal-organic frameworks (MOFs) have attracted overwhelming attention. With readily tunable structures and functionalities, MOFs offer an unprecedentedly vast degree of design flexibility from enormous number of inorganic and organic building blocks or via postsynthetic modification to produce functional nanoporous materials. A large extent of experimental and computational studies of MOFs have been focused on gas phase applications, particularly the storage of low-carbon footprint energy carriers and the separation of CO2-containing gas mixtures. With progressive success in the synthesis of water- and solvent-resistant MOFs over the past several years, the increasingly active exploration of MOFs has been witnessed for widespread liquid phase applications such as liquid fuel purification, aromatics separation, water treatment, solvent recovery, chemical sensing, chiral separation, drug delivery, biomolecule encapsulation and separation. At this juncture, the recent experimental and computational studies are summarized herein for these multifaceted liquid phase applications to demonstrate the rapid advance in this burgeoning field. The challenges and opportunities moving from laboratory scale towards practical applications are discussed.
Keywords: computations; liquid phase applications; metal−organic frameworks; nanoporous materials.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




































Similar articles
-
Postsynthetic Tuning of Metal-Organic Frameworks for Targeted Applications.Acc Chem Res. 2017 Apr 18;50(4):805-813. doi: 10.1021/acs.accounts.6b00577. Epub 2017 Feb 8. Acc Chem Res. 2017. PMID: 28177217
-
[Application of gas chromatography separation based on metal-organic framework material as stationary phase].Se Pu. 2021 Jan;39(1):57-68. doi: 10.3724/SP.J.1123.2020.06028. Se Pu. 2021. PMID: 34227359 Free PMC article. Chinese.
-
[Synthesis of porous organic framework materials based on deep eutectic solvents and their application in solid-phase extraction].Se Pu. 2023 Oct;41(10):901-910. doi: 10.3724/SP.J.1123.2023.08025. Se Pu. 2023. PMID: 37875412 Free PMC article. Chinese.
-
State-of-the-Art Advances and Challenges of Iron-Based Metal Organic Frameworks from Attractive Features, Synthesis to Multifunctional Applications.Small. 2019 Jan;15(2):e1803088. doi: 10.1002/smll.201803088. Epub 2018 Dec 7. Small. 2019. PMID: 30548176 Review.
-
Metal-organic frameworks as stationary phase for application in chromatographic separation.J Chromatogr A. 2017 Dec 29;1530:1-18. doi: 10.1016/j.chroma.2017.10.065. Epub 2017 Oct 27. J Chromatogr A. 2017. PMID: 29150064 Review.
Cited by
-
Unravelling nonclassical beam damage mechanisms in metal-organic frameworks by low-dose electron microscopy.Nat Commun. 2025 Jan 2;16(1):261. doi: 10.1038/s41467-024-55632-w. Nat Commun. 2025. PMID: 39747904 Free PMC article.
-
Utilizing EPR spectroscopy to investigate the liquid adsorption properties of bimetallic MIL-53(Al/Cr) MOF.RSC Adv. 2024 Jan 30;14(6):4244-4251. doi: 10.1039/d3ra07952j. eCollection 2024 Jan 23. RSC Adv. 2024. PMID: 38292261 Free PMC article.
-
Biomimetic and bioorthogonal nanozymes for biomedical applications.Nano Converg. 2023 Sep 11;10(1):42. doi: 10.1186/s40580-023-00390-6. Nano Converg. 2023. PMID: 37695365 Free PMC article. Review.
-
Unveiling the MIL-53(Al) MOF: Tuning Photoluminescence and Structural Properties via Volatile Organic Compounds Interactions.Nanomaterials (Basel). 2024 Feb 20;14(5):388. doi: 10.3390/nano14050388. Nanomaterials (Basel). 2024. PMID: 38470719 Free PMC article.
-
Synthesis and characterization of tetraethylene pentamine functionalized MIL-101(Cr) for removal of metals from water.J Environ Health Sci Eng. 2021 Sep 1;19(2):1735-1742. doi: 10.1007/s40201-021-00728-4. eCollection 2021 Dec. J Environ Health Sci Eng. 2021. PMID: 34900302 Free PMC article.
References
-
- Ishizaki K., Komarneni S., Nanko M., Porous Materials, Springer, Boston, MA: 1998.
-
- Baerlocher C., McCusker L. B., Database of Zeolite Structures , http://www.iza-structure.org/databases/
-
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M., Science 2013, 341, 1230444. - PubMed
-
- https://www.ccdc.cam.ac.uk/ (accessed: August 2020).
-
- Ferey G., Chem. Soc. Rev. 2008, 37, 191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
