A screening approach for the evaluation of tobacco-free 'modern oral' nicotine products using Real Time Cell Analysis
- PMID: 33718000
- PMCID: PMC7933807
- DOI: 10.1016/j.toxrep.2021.02.014
A screening approach for the evaluation of tobacco-free 'modern oral' nicotine products using Real Time Cell Analysis
Abstract
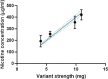
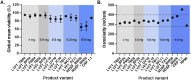
In many regulated industries there is an increasing pressure to provide timely and robust risk assessment data to support product launches. Real-time cell analysis (RTCA) is a tool that allows for the fast and relatively labour-free cytotoxic assessment of test compounds, compared to traditional methods. Here, we propose an application for the RTCA platform to provide a screening approach, to evaluate the cytotoxic potential of tobacco-free nicotine pouches, also termed modern oral product (MOP), to determine the contribution of differing nicotine strengths (4-11 mg) and a range of available flavour types from multiple markets, on overall product toxicity. Aqueous extracts were prepared for all products using 1 pouch in 20 mL cell culture media and applied to the cell system for 24 h. Test extract nicotine concentrations reflected the increases in product nicotine strength; however, these changes were not present in the same magnitude in the cytotoxicity data obtained from both primary human gingival fibroblasts (HGF) and an NCI-H292 human bronchial epithelial continuous cell line. Furthermore, across the range of flavours and product nicotine strengths tested, H292 cells whilst not the target organ for oral product use, accurately predicted the results seen in HGFs and could be considered a useful surrogate for fast screening studies. H292 cells are more easily cultured and for longer periods, offering a more compatible test system. In conclusion, the data demonstrate the utility of the RTCA platform for the quick assessment of a large range of product variants. Furthermore, for a cytotoxicity measure with this test product, the simple H292 cell line can predict outcomes in the more complex HGF and provide useful pre-clinical cytotoxicity screening data to inform the risk assessment of MOPs and the relative contribution of flavourings, nicotine and other components.
Keywords: AqE, Aqueous extract; CRP, 1.1 CORESTA Reference Product 1.1; Cytotoxicity; H292, Human bronchial epithelial cells; HGF, Human gingival fibroblasts; In vitro; LDH, Lactate dehydrogenase assay; MOP, Modern oral product; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRU, Neutral red uptake assay; Nicotine; RTCA; RTCA, Real Time Cell Analysis; Risk assessment; Tobacco-free modern oral tobacco; Tobacco-free nicotine pouches.
© 2021 British American Tobacco.
Conflict of interest statement
BAT funded the project and all authors were employees of BAT at the time of the study conduct. LYFT tobacco-free modern oral nicotine pouches are manufactured and supplied by BAT.
Figures




Similar articles
-
An approach for the extract generation and toxicological assessment of tobacco-free 'modern' oral nicotine pouches.Food Chem Toxicol. 2020 Nov;145:111713. doi: 10.1016/j.fct.2020.111713. Epub 2020 Sep 28. Food Chem Toxicol. 2020. PMID: 32998027
-
Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells.Toxics. 2022 Oct 31;10(11):660. doi: 10.3390/toxics10110660. Toxics. 2022. PMID: 36355951 Free PMC article.
-
Multi-endpoint in vitro toxicological assessment of snus and tobacco-free nicotine pouch extracts.Mutat Res Genet Toxicol Environ Mutagen. 2024 Apr;895:503738. doi: 10.1016/j.mrgentox.2024.503738. Epub 2024 Feb 20. Mutat Res Genet Toxicol Environ Mutagen. 2024. PMID: 38575247
-
Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. A systematic review of the literature.J Dent. 2019 Jul;86:81-88. doi: 10.1016/j.jdent.2019.05.030. Epub 2019 May 25. J Dent. 2019. PMID: 31136818
-
Emerging Oral Nicotine Products and Periodontal Diseases.Int J Dent. 2023 Feb 10;2023:9437475. doi: 10.1155/2023/9437475. eCollection 2023. Int J Dent. 2023. PMID: 36819641 Free PMC article. Review.
Cited by
-
Tobacco-Free Nicotine Pouches and Their Potential Contribution to Tobacco Harm Reduction: A Scoping Review.Cureus. 2024 Feb 15;16(2):e54228. doi: 10.7759/cureus.54228. eCollection 2024 Feb. Cureus. 2024. PMID: 38496069 Free PMC article.
-
The Potential Impact of Oral Nicotine Pouches on Public Health: A Scoping Review.Nicotine Tob Res. 2025 Mar 24;27(4):598-610. doi: 10.1093/ntr/ntae131. Nicotine Tob Res. 2025. PMID: 38880491 Free PMC article.
-
Oral nicotine pouches with an aftertaste? Part 2: in vitro toxicity in human gingival fibroblasts.Arch Toxicol. 2023 Sep;97(9):2343-2356. doi: 10.1007/s00204-023-03554-9. Epub 2023 Jul 23. Arch Toxicol. 2023. PMID: 37482550 Free PMC article.
-
Bridging: Accelerating Regulatory Acceptance of Reduced-Risk Tobacco and Nicotine Products.Nicotine Tob Res. 2022 Aug 6;24(9):1371-1378. doi: 10.1093/ntr/ntac041. Nicotine Tob Res. 2022. PMID: 35171296 Free PMC article. Review.
-
In vitro toxicological evaluation of pouched portioned oral nicotine products.Front Toxicol. 2024 Nov 28;6:1452274. doi: 10.3389/ftox.2024.1452274. eCollection 2024. Front Toxicol. 2024. PMID: 39669663 Free PMC article.
References
-
- Lee P.N. Summary of the epidemiological evidence relating snus to health. Regul. Toxicol. Pharmacol. 2011;59:197–214. - PubMed
-
- Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. - PubMed
-
- Roosaar A., Johansson A.L., Sandborgh-Englund G., Nyrén O., Axéll T. A long-term follow-up study on the natural course of snus-induced lesions among Swedish snus users. Int. J. Cancer. 2006;119:392–397. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

