KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma
- PMID: 33718018
- PMCID: PMC7947421
- DOI: 10.21037/tlcr-20-958
KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma
Abstract
Background: Pembrolizumab is a standard of care as first line palliative therapy in PD-L1 overexpressing (≥50%) non-small cell lung cancer (NSCLC). This study aimed at the identification of KRAS and TP53-defined mutational subgroups in the PD-L1 high population to distinguish long-term responders from those with limited benefit.
Methods: In this retrospective, observational study, patients from 4 certified lung cancer centers in Berlin, Germany, having received pembrolizumab monotherapy as first line palliative treatment for lung adenocarcinoma (LuAD) from 2017 to 2018, with PD-L1 expression status and targeted NGS data available, were evaluated.
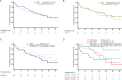
Results: A total of 119 patients were included. Rates for KRAS, TP53 and combined mutations were 52.1%, 47.1% and 21.9%, respectively, with no association given between KRAS and TP53 mutations (P=0.24). By trend, PD-L1 expression was higher in KRAS-positive patients (75% vs. 65%, P=0.13). Objective response rate (ORR), median progression-free survival (PFS) and overall survival (OS) in the KRASG12C group (n=32, 51.6%) were 63.3%, 19.8 months (mo.) and not estimable (NE), respectively. Results in KRASother and wild type patients were similar and by far lower (42.7%, P=0.06; 6.2 mo., P<0.001; 23.4 mo., P=0.08). TP53 mutations alone had no impact on response and survival. However, KRASG12C/TP53 co-mutations (n=12) defined a subset of long-term responders (ORR 100.0%, PFS 33.3 mo., OS NE). In contrast, patients with KRASother/TP53 mutations showed a dismal prognosis (ORR 27.3%, P=0.002; PFS 3.9 mo., P=0.001, OS 9.7 mo., P=0.02).
Conclusions: A comprehensive assessment of KRAS subtypes and TP53 mutations allows a highly relevant prognostic differentiation of patients with metastatic, PD-L1 high LuAD treated upfront with pembrolizumab.
Keywords: KRAS mutations; Non-small cell lung cancer (NSCLC); TP53 mutations; checkpoint inhibitors.
2021 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-958). NF reports personal fees and other from AstraZeneca, personal fees and other from Bristol Myers Squibb, personal fees and other from AbbVie, personal fees and other from Boehringer Ingelheim, personal fees from Pfizer, personal fees from Roche Pharma, personal fees from Merck Sharp & Dohme, personal fees from Takeda, all outside the submitted work. JK reports being an advisory Board member without receiving any personal fees for: Roche Pharma, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Takeda and Lilly Oncology, all outside the submitted work. The other authors have no conflicts of interest to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
