m6A Modifications Play Crucial Roles in Glial Cell Development and Brain Tumorigenesis
- PMID: 33718165
- PMCID: PMC7943831
- DOI: 10.3389/fonc.2021.611660
m6A Modifications Play Crucial Roles in Glial Cell Development and Brain Tumorigenesis
Abstract
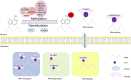
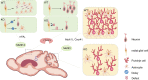
RNA methylation is a reversible post-transcriptional modification to RNA and has a significant impact on numerous biological processes. N 6-methyladenosine (m6A) is known as one of the most common types of eukaryotic mRNA methylation modifications, and exists in a wide variety of organisms, including viruses, yeast, plants, mice, and humans. Widespread and dynamic m6A methylation is identified in distinct developmental stages in the brain, and controls development of neural stem cells and their differentiation into neurons, glial cells such as oligodendrocytes and astrocytes. Here we summarize recent advances in our understanding of RNA methylation regulation in brain development, neurogenesis, gliogenesis, and its dysregulation in brain tumors. This review will highlight biological roles of RNA methylation in development and function of neurons and glial cells, and provide insights into brain tumor formation, and diagnostic and treatment strategies.
Keywords: N6-methyladenosine (m6A); brain development; brain tumor; glial cell; glioma; neural stem cell.
Copyright © 2021 Wang, Sha and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The role of mRNA m6A methylation in the nervous system.Cell Biosci. 2019 Aug 20;9:66. doi: 10.1186/s13578-019-0330-y. eCollection 2019. Cell Biosci. 2019. PMID: 31452869 Free PMC article. Review.
-
Functions of RNA N6-methyladenosine modification in acute myeloid leukemia.Biomark Res. 2021 May 17;9(1):36. doi: 10.1186/s40364-021-00293-w. Biomark Res. 2021. PMID: 34001273 Free PMC article. Review.
-
Recent Advances in RNA m6A Modification in Solid Tumors and Tumor Immunity.Cancer Treat Res. 2023;190:95-142. doi: 10.1007/978-3-031-45654-1_4. Cancer Treat Res. 2023. PMID: 38113000
-
N6-methyladenine RNA modification and cancer.Oncol Lett. 2020 Aug;20(2):1504-1512. doi: 10.3892/ol.2020.11739. Epub 2020 Jun 16. Oncol Lett. 2020. PMID: 32724392 Free PMC article. Review.
-
RNA methylation in mammalian development and cancer.Cell Biol Toxicol. 2021 Dec;37(6):811-831. doi: 10.1007/s10565-021-09627-8. Epub 2021 Jul 17. Cell Biol Toxicol. 2021. PMID: 34272618 Free PMC article. Review.
Cited by
-
MYC-activated RNA N6-methyladenosine reader IGF2BP3 promotes cell proliferation and metastasis in nasopharyngeal carcinoma.Cell Death Discov. 2022 Feb 8;8(1):53. doi: 10.1038/s41420-022-00844-6. Cell Death Discov. 2022. PMID: 35136045 Free PMC article.
-
m6A methylation: Critical roles in aging and neurological diseases.Front Mol Neurosci. 2023 Feb 21;16:1102147. doi: 10.3389/fnmol.2023.1102147. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36896007 Free PMC article. Review.
-
The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction.Antioxidants (Basel). 2022 Aug 4;11(8):1521. doi: 10.3390/antiox11081521. Antioxidants (Basel). 2022. PMID: 36009239 Free PMC article. Review.
-
N6-Methyladenosine Methylation of mRNA in Cell Senescence.Cell Mol Neurobiol. 2023 Jan;43(1):27-36. doi: 10.1007/s10571-021-01168-2. Epub 2021 Nov 12. Cell Mol Neurobiol. 2023. PMID: 34767142 Free PMC article. Review.
-
Differential Co-Expression Analysis of RNA-Seq Data Reveals Novel Potential Biomarkers of Device-Tissue Interaction.Annu Int Conf IEEE Eng Med Biol Soc. 2022 Jul;2022:3072-3076. doi: 10.1109/EMBC48229.2022.9871437. Annu Int Conf IEEE Eng Med Biol Soc. 2022. PMID: 36085767 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

