Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers
- PMID: 33718169
- PMCID: PMC7947611
- DOI: 10.3389/fonc.2021.614332
Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers
Abstract
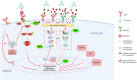
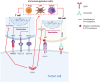
Head and neck squamous cell carcinoma (HNSCC) is the sixth most incident cancer worldwide. More than half of HNSCC patients experience locoregional or distant relapse to treatment despite aggressive multimodal therapeutic approaches that include surgical resection, radiation therapy, and adjuvant chemotherapy. Before the arrival of immunotherapy, systemic chemotherapy was previously employed as the standard first-line protocol with an association of cisplatin or carboplatin plus 5-fluorouracil plus cetuximab (anti-EFGR antibody). Unfortunately, acquisition of therapy resistance is common in patients with HNSCC and often results in local and distant failure. Despite our better understanding of HNSCC biology, no other molecular-targeted agent has been approved for HNSCC. In this review, we outline the mechanisms of resistance to the therapeutic strategies currently used in HNSCC, discuss combination treatment strategies to overcome them, and summarize the therapeutic regimens that are presently being evaluated in early- and late-phase clinical trials.
Keywords: cetuximab; chemotherapy; head and neck squamous-cell carcinoma; immunotherapy; resistance; targeted therapy.
Copyright © 2021 Ortiz-Cuaran, Bouaoud, Karabajakian, Fayette and Saintigny.
Conflict of interest statement
PS receives research grants from HTG Molecular Diagnostics, Inivata, Bristol-Myers Squibb, Roche Molecular Diagnostics, Roche, AstraZeneca, Novartis, Bristol-Myers Squibb Foundation, and Illumina. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma.Front Oncol. 2019 May 20;9:383. doi: 10.3389/fonc.2019.00383. eCollection 2019. Front Oncol. 2019. PMID: 31165040 Free PMC article. Review.
-
Targeted radionuclide therapy for head and neck squamous cell carcinoma: a review.Front Oncol. 2024 Aug 22;14:1445191. doi: 10.3389/fonc.2024.1445191. eCollection 2024. Front Oncol. 2024. PMID: 39239273 Free PMC article. Review.
-
Immunotherapy in Patients with Recurrent and Metastatic Squamous Cell Carcinoma of the Head and Neck.Anticancer Agents Med Chem. 2019;19(3):290-303. doi: 10.2174/1871520618666180910092356. Anticancer Agents Med Chem. 2019. PMID: 30198439 Review.
-
Docetaxel plus cisplatin plus fluorouracil versus carboplatin plus fluorouracil-cetuximab in first-line setting in patients with recurrent or metastatic head and neck squamous cell cancer who did not previously receive neoadjuvant or adjuvant chemotherapy, which is standard?J Cancer Res Ther. 2017 Jul-Sep;13(3):510-513. doi: 10.4103/0973-1482.161933. J Cancer Res Ther. 2017. PMID: 28862218
-
Current Prospects of Molecular Therapeutics in Head and Neck Squamous Cell Carcinoma.Pharmaceut Med. 2019 Aug;33(4):269-289. doi: 10.1007/s40290-019-00288-x. Pharmaceut Med. 2019. PMID: 31933185 Review.
Cited by
-
The Role of Hedgehog Signaling Pathway in Head and Neck Squamous Cell Carcinoma.Cells. 2023 Aug 17;12(16):2083. doi: 10.3390/cells12162083. Cells. 2023. PMID: 37626893 Free PMC article. Review.
-
Long Intergenic Non-Coding RNAs in HNSCC: From "Junk DNA" to Important Prognostic Factor.Cancers (Basel). 2021 Jun 12;13(12):2949. doi: 10.3390/cancers13122949. Cancers (Basel). 2021. PMID: 34204634 Free PMC article. Review.
-
Current innovations in head and neck cancer: From diagnostics to therapeutics.Oncol Res. 2025 Apr 18;33(5):1019-1032. doi: 10.32604/or.2025.060601. eCollection 2025. Oncol Res. 2025. PMID: 40296914 Free PMC article. Review.
-
Inhibiting the inhibitors: Development of the IAP inhibitor xevinapant for the treatment of locally advanced squamous cell carcinoma of the head and neck.Cancer Treat Rev. 2023 Feb;113:102492. doi: 10.1016/j.ctrv.2022.102492. Epub 2022 Nov 30. Cancer Treat Rev. 2023. PMID: 36640618 Free PMC article. Review.
-
Cancer Stem Cells in Oral Squamous Cell Carcinoma: A Narrative Review on Experimental Characteristics and Methodological Challenges.Biomedicines. 2024 Sep 16;12(9):2111. doi: 10.3390/biomedicines12092111. Biomedicines. 2024. PMID: 39335624 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

