Glioma Stem Cells as Immunotherapeutic Targets: Advancements and Challenges
- PMID: 33718170
- PMCID: PMC7945033
- DOI: 10.3389/fonc.2021.615704
Glioma Stem Cells as Immunotherapeutic Targets: Advancements and Challenges
Abstract
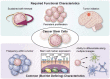
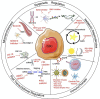
Glioblastoma is the most common and lethal primary brain malignancy. Despite major investments in research into glioblastoma biology and drug development, treatment remains limited and survival has not substantially improved beyond 1-2 years. Cancer stem cells (CSC) or glioma stem cells (GSC) refer to a population of tumor originating cells capable of self-renewal and differentiation. While controversial and challenging to study, evidence suggests that GCSs may result in glioblastoma tumor recurrence and resistance to treatment. Multiple treatment strategies have been suggested at targeting GCSs, including immunotherapy, posttranscriptional regulation, modulation of the tumor microenvironment, and epigenetic modulation. In this review, we discuss recent advances in glioblastoma treatment specifically focused on targeting of GCSs as well as their potential integration into current clinical pathways and trials.
Keywords: brain tumors; cancer stem cell; cancer vaccination; glioblastoma; glioblastoma stem cells; immunotherapy; radioresistance; tumor recurrence.
Copyright © 2021 Piper, DePledge, Karsy and Cobbs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Glioma Stem Cells: Signaling, Microenvironment, and Therapy.Stem Cells Int. 2016;2016:7849890. doi: 10.1155/2016/7849890. Epub 2016 Jan 6. Stem Cells Int. 2016. PMID: 26880988 Free PMC article. Review.
-
Immunotherapy targeting glioma stem cells--insights and perspectives.Expert Opin Biol Ther. 2012 Feb;12(2):165-78. doi: 10.1517/14712598.2012.648180. Epub 2011 Dec 26. Expert Opin Biol Ther. 2012. PMID: 22200324 Review.
-
Impairing temozolomide resistance driven by glioma stem-like cells with adjuvant immunotherapy targeting O-acetyl GD2 ganglioside.Int J Cancer. 2020 Jan 15;146(2):424-438. doi: 10.1002/ijc.32533. Epub 2019 Jul 13. Int J Cancer. 2020. PMID: 31241171
-
Chromatin remodeler HELLS maintains glioma stem cells through E2F3 and MYC.JCI Insight. 2019 Apr 4;4(7):e126140. doi: 10.1172/jci.insight.126140. eCollection 2019 Apr 4. JCI Insight. 2019. PMID: 30779712 Free PMC article.
-
Potential Therapeutic Effects of the Neural Stem Cell-Targeting Antibody Nilo1 in Patient-Derived Glioblastoma Stem Cells.Front Oncol. 2020 Aug 14;10:1665. doi: 10.3389/fonc.2020.01665. eCollection 2020. Front Oncol. 2020. PMID: 32974206 Free PMC article.
Cited by
-
Pathogenesis Study of Glioma: From Glioma Stem Cells, Genomic Tags, to Rodent Models.Brain Sci. 2022 Dec 23;13(1):30. doi: 10.3390/brainsci13010030. Brain Sci. 2022. PMID: 36672013 Free PMC article.
-
MDM2/X Inhibitors as Radiosensitizers for Glioblastoma Targeted Therapy.Front Oncol. 2021 Jul 8;11:703442. doi: 10.3389/fonc.2021.703442. eCollection 2021. Front Oncol. 2021. PMID: 34307171 Free PMC article. Review.
-
Independently validated sex-specific nomograms for predicting survival in patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825.J Neurooncol. 2021 Dec;155(3):363-372. doi: 10.1007/s11060-021-03886-5. Epub 2021 Nov 10. J Neurooncol. 2021. PMID: 34761331 Free PMC article.
-
Insights of immune cell heterogeneity, tumor-initiated subtype transformation, drug resistance, treatment and detecting technologies in glioma microenvironment.J Adv Res. 2025 Jun;72:527-554. doi: 10.1016/j.jare.2024.07.033. Epub 2024 Aug 5. J Adv Res. 2025. PMID: 39097088 Free PMC article. Review.
-
Interdependencies of the Neuronal, Immune and Tumor Microenvironment in Gliomas.Cancers (Basel). 2023 May 21;15(10):2856. doi: 10.3390/cancers15102856. Cancers (Basel). 2023. PMID: 37345193 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

