Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies?
- PMID: 33718177
- PMCID: PMC7943718
- DOI: 10.3389/fonc.2021.620773
Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies?
Abstract
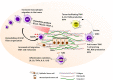
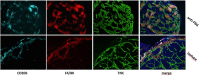
Solid cancers such as breast tumors comprise a collection of tumor, stromal and immune cells, embedded within a network of tumor-specific extracellular matrix. This matrix is associated with tumor aggression, treatment failure, chemo- and radio-resistance, poor survival and metastasis. Recent data report an immunomodulatory role for the matrix in cancer, via the creation of niches that control the migration, localization, phenotype and function of tumor-infiltrating immune cells, ultimately contributing to escape of immune surveillance. Macrophages are crucial components of the immune infiltrate in tumors; they are associated with a poor prognosis in breast cancer and contribute to shaping the anti-tumor immune response. We and others have described how matrix molecules commonly upregulated within the tumor stroma, such as tenascin-C, fibronectin and collagen, exert a complex influence over macrophage behavior, for example restricting or enhancing their infiltration into the tumor, and driving their polarization towards or away from a pro-tumoral phenotype, and how in turn macrophages can modify matrix production in the tumor to favor tumor growth and metastasis. Targeting specific domains of matrix molecules to reinstate an efficient anti-tumor immune response, and effectively control tumor growth and spread, is emerging as a promising field offering a new angle for cancer therapy. Here, we review current knowledge on the interactions between tumor-associated macrophages and matrix molecules that occur within the tumor microenvironment of breast cancer, and discuss how these pathways can be targeted for new immunotherapies for hard to treat, desmoplastic tumors.
Keywords: breast cancer; extracellular matrix; immune infiltrate; immunotherapy; macrophages; tumor microenvironment.
Copyright © 2021 Deligne and Midwood.
Conflict of interest statement
KM is founder of, and consultant to, Nascient Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Matrix-Targeting Immunotherapy Controls Tumor Growth and Spread by Switching Macrophage Phenotype.Cancer Immunol Res. 2020 Mar;8(3):368-382. doi: 10.1158/2326-6066.CIR-19-0276. Epub 2020 Jan 15. Cancer Immunol Res. 2020. PMID: 31941671 Free PMC article.
-
The role of tumor-associated macrophage in breast cancer biology.Histol Histopathol. 2018 Feb;33(2):133-145. doi: 10.14670/HH-11-916. Epub 2017 Jul 6. Histol Histopathol. 2018. PMID: 28681373 Review.
-
The metabolic axis of macrophage and immune cell polarization.Dis Model Mech. 2018 Jul 6;11(8):dmm034462. doi: 10.1242/dmm.034462. Dis Model Mech. 2018. PMID: 29991530 Free PMC article. Review.
-
Targeting Macrophages in Cancer: From Bench to Bedside.Front Oncol. 2018 Mar 12;8:49. doi: 10.3389/fonc.2018.00049. eCollection 2018. Front Oncol. 2018. PMID: 29594035 Free PMC article. Review.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
Adipose tissue radiodensity and mortality among patients with nonmetastatic breast cancer.Clin Nutr. 2022 Dec;41(12):2607-2613. doi: 10.1016/j.clnu.2022.09.016. Epub 2022 Oct 4. Clin Nutr. 2022. PMID: 36306565 Free PMC article.
-
Decoding the multicellular ecosystem of vena caval tumor thrombus in clear cell renal cell carcinoma by single-cell RNA sequencing.Genome Biol. 2022 Mar 31;23(1):87. doi: 10.1186/s13059-022-02651-9. Genome Biol. 2022. PMID: 35361264 Free PMC article.
-
Defining and targeting macrophage heterogeneity in the mammary gland and breast cancer.Cancer Med. 2024 Feb;13(3):e7053. doi: 10.1002/cam4.7053. Cancer Med. 2024. PMID: 38426622 Free PMC article. Review.
-
Transcriptomics analysis reveals distinct mechanism of breast cancer stem cells regulation in mammospheres from MCF-7 and T47D cells.Heliyon. 2024 Jan 12;10(2):e24356. doi: 10.1016/j.heliyon.2024.e24356. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38304813 Free PMC article.
-
Extracellular Matrix-Based Gene Expression Signature Defines Two Prognostic Subtypes of Hepatocellular Carcinoma With Different Immune Microenvironment Characteristics.Front Mol Biosci. 2022 Mar 25;9:839806. doi: 10.3389/fmolb.2022.839806. eCollection 2022. Front Mol Biosci. 2022. PMID: 35402515 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

