Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes
- PMID: 33718188
- PMCID: PMC7947882
- DOI: 10.3389/fonc.2021.624742
Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes
Abstract
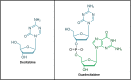
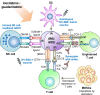
Decitabine and guadecitabine are hypomethylating agents (HMAs) that exert inhibitory effects against cancer cells. This includes stimulation of anti-tumor immunity in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) patients. Treatment of AML and MDS patients with the HMAs confers upregulation of cancer/testis antigens (CTAs) expression including the highly immunogenic CTA NY-ESO-1. This leads to activation of CD4+ and CD8+ T cells for elimination of cancer cells, and it establishes the feasibility to combine cancer vaccine with HMAs to enhance vaccine immunogenicity. Moreover, decitabine and guadecitabine induce the expression of immune checkpoint molecules in AML cells. In this review, the accumulating knowledge on the immunopotentiating properties of decitabine and guadecitabine in AML and MDS patients are presented and discussed. In summary, combination of decitabine or guadecitabine with NY-ESO-1 vaccine enhances vaccine immunogenicity in AML patients. T cells from AML patients stimulated with dendritic cell (DC)/AML fusion vaccine and guadecitabine display increased capacity to lyse AML cells. Moreover, decitabine enhances NK cell-mediated cytotoxicity or CD123-specific chimeric antigen receptor-engineered T cells antileukemic activities against AML. Furthermore, combination of either HMAs with immune checkpoint blockade (ICB) therapy may circumvent their resistance. Finally, clinical trials of either HMAs combined with cancer vaccines, NK cell infusion or ICB therapy in relapsed/refractory AML and high-risk MDS patients are currently underway, highlighting the promising efficacy of HMAs and immunotherapy synergy against these malignancies.
Keywords: acute myeloid leukemia; cancer vaccine; chimeric antigen receptor-engineered (CAR)-T cell therapy; hypomethylating agents; immune checkpoint; myelodysplastic syndromes.
Copyright © 2021 Wong, Hassan and Yaacob.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-lymphocyte Responses in Patients with Myelodysplastic Syndrome.Clin Cancer Res. 2018 Mar 1;24(5):1019-1029. doi: 10.1158/1078-0432.CCR-17-1792. Epub 2017 Sep 25. Clin Cancer Res. 2018. PMID: 28947565 Free PMC article. Clinical Trial.
-
Hypomethylating agent alters the immune microenvironment in acute myeloid leukaemia (AML) and enhances the immunogenicity of a dendritic cell/AML vaccine.Br J Haematol. 2019 May;185(4):679-690. doi: 10.1111/bjh.15818. Epub 2019 Mar 3. Br J Haematol. 2019. PMID: 30828801 Free PMC article.
-
Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy.Oncotarget. 2016 Mar 15;7(11):12840-56. doi: 10.18632/oncotarget.7326. Oncotarget. 2016. PMID: 26883197 Free PMC article.
-
Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes.Leukemia. 2018 May;32(5):1094-1105. doi: 10.1038/s41375-018-0070-8. Epub 2018 Feb 22. Leukemia. 2018. PMID: 29487386 Free PMC article. Review.
-
Guadecitabine (SGI-110): an investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia.Expert Opin Investig Drugs. 2019 Oct;28(10):835-849. doi: 10.1080/13543784.2019.1667331. Epub 2019 Sep 19. Expert Opin Investig Drugs. 2019. PMID: 31510809 Review.
Cited by
-
Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment.Metabolites. 2023 Jan 13;13(1):123. doi: 10.3390/metabo13010123. Metabolites. 2023. PMID: 36677048 Free PMC article.
-
Clinical characteristics, treatment, and prognosis of 118 cases of myeloid sarcoma.Sci Rep. 2022 Apr 26;12(1):6752. doi: 10.1038/s41598-022-10831-7. Sci Rep. 2022. PMID: 35474239 Free PMC article.
-
Transcriptomic analysis of non-leukemic cell subsets in azacytidine-responsive AML highlights pathways associated with adhesion, platelet aggregation, and angiogenesis in mice and humans.Mol Med. 2025 May 13;31(1):185. doi: 10.1186/s10020-025-01233-2. Mol Med. 2025. PMID: 40360989 Free PMC article.
-
Prospects and feasibility of synergistic therapy with radiotherapy, immunotherapy, and DNA methyltransferase inhibitors in non-small cell lung cancer.Front Immunol. 2023 Feb 17;14:1122352. doi: 10.3389/fimmu.2023.1122352. eCollection 2023. Front Immunol. 2023. PMID: 36875059 Free PMC article. Review.
-
Efficacy of Cemiplimab in a Patient Affected by Cutaneous Squamous Cell Carcinoma and Myelodysplastic Syndrome.Dermatol Pract Concept. 2023 Jul 1;13(3):e2023178. doi: 10.5826/dpc.1303a178. Dermatol Pract Concept. 2023. PMID: 37557104 Free PMC article. No abstract available.
References
-
- Hulegardh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf A, et al. . Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol (2015) 90:208–14. 10.1002/ajh.23908 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

