The Impact of Epstein-Barr Virus Infection on Epigenetic Regulation of Host Cell Gene Expression in Epithelial and Lymphocytic Malignancies
- PMID: 33718209
- PMCID: PMC7947917
- DOI: 10.3389/fonc.2021.629780
The Impact of Epstein-Barr Virus Infection on Epigenetic Regulation of Host Cell Gene Expression in Epithelial and Lymphocytic Malignancies
Abstract
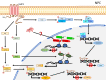
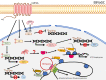
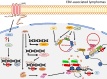
Epstein-Barr virus (EBV) infection is associated with a variety of malignancies including Burkitt's lymphoma (BL), Hodgkin's disease, T cell lymphoma, nasopharyngeal carcinoma (NPC), and ∼10% of cases of gastric cancer (EBVaGC). Disruption of epigenetic regulation in the expression of tumor suppressor genes or oncogenes has been considered as one of the important mechanisms for carcinogenesis. Global hypermethylation is a distinct feature in NPC and EBVaGC, whereas global reduction of H3K27me3 is more prevalent in EBVaGC and EBV-transformed lymphoblastoid cells. In BL, EBV may even usurp the host factors to epigenetically regulate its own viral gene expression to restrict latency and lytic switch, resulting in evasion of immunosurveillance. Furthermore, in BL and EBVaGC, the interaction between the EBV episome and the host genome is evident with respectively unique epigenetic features. While the interaction is associated with suppression of gene expression in BL, the corresponding activity in EBVaGC is linked to activation of gene expression. As EBV establishes a unique latency program in these cancer types, it is possible that EBV utilizes different latency proteins to hijack the epigenetic modulators in the host cells for pathogenesis. Since epigenetic regulation of gene expression is reversible, understanding the precise mechanisms about how EBV dysregulates the epigenetic mechanisms enables us to identify the potential targets for epigenetic therapies. This review summarizes the currently available epigenetic profiles of several well-studied EBV-associated cancers and the relevant distinct mechanisms leading to aberrant epigenetic signatures due to EBV.
Keywords: DNA methylation; EBV-associated gastric cancer; EBV-associated lymphomas; Epstein-Barr virus; epigenetics; histone modifications; nasopharyngeal carcinoma.
Copyright © 2021 Leong and Lung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

