Curdione Induces Antiproliferation Effect on Human Uterine Leiomyosarcoma via Targeting IDO1
- PMID: 33718227
- PMCID: PMC7953905
- DOI: 10.3389/fonc.2021.637024
Curdione Induces Antiproliferation Effect on Human Uterine Leiomyosarcoma via Targeting IDO1
Abstract
Objectives: Curdione is one of the active ingredients of a traditional Chinese herbal medicine-Curcuma zedoary and established anti-tumor effects. Uterine leiomyosarcoma (uLMS) is a rare gynecological malignancy, with no standard therapeutic regimen at present. The aim of this study was to explore the potential anti-tumor impact of curdione in uLMS and elucidate the underlying mechanisms.
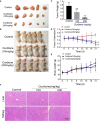
Methods: In vitro functional assays were performed in the SK-UT-1 and SK-LMS-1 cell lines. The in vivo model of uLMS was established by subcutaneously injecting SK-UT-1 cells, and the tumor-bearing mice were intraperitoneally injected with curdione. Tumor weight and volume were measured at specific time points. The biosafety was evaluated by monitoring changes of body weight and the histopathology in the liver and kidney. The expression levels of relevant proteins were analyzed by western blotting and immunohistochemistry.
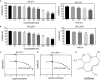
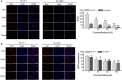
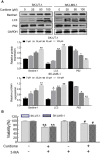
Results: Curdione decreased the viability and proliferation of uLMS cells in a concentration and time-dependent manner. In addition, the curdione-treated cells exhibited significantly higher rates of apoptosis and autophagic death. Curdione also decreased the tumor weight and volume in the SK-UT-1 xenograft model compared to the untreated control without affecting the body bodyweight or pathological injury of liver and kidney tissues. At the molecular level, the anti-tumor effects of curdione were mediated by indoleamine-2, 3-dioxygenase-1 (IDO1).
Conclusion: Curdione exhibited an anti-uLMS effect in vitro and in vivo; the underlying mechanism involved in IDO1 mediate apoptosis, autophagy, and G2/M phase arrest.
Keywords: Indoleamine-2, 3-dioxygenase-1; apoptosis; autophagy; curdione; uterine leiomyosarcoma.
Copyright © 2021 Wei, Li, Liu, Wang and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1.Cancers (Basel). 2022 Sep 12;14(18):4424. doi: 10.3390/cancers14184424. Cancers (Basel). 2022. PMID: 36139584 Free PMC article.
-
Gamma Secretase Inhibitors as Potential Therapeutic Targets for Notch Signaling in Uterine Leiomyosarcoma.Int J Mol Sci. 2022 May 26;23(11):5980. doi: 10.3390/ijms23115980. Int J Mol Sci. 2022. PMID: 35682660 Free PMC article.
-
A nutrient mixture reduced tumor growth of SK-UT-1 human leiomyosarcoma cells in vivo and in vitro by inhibiting MMPs and inducing apoptosis.Exp Oncol. 2021 Sep;43(3):209-216. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-3.16604. Exp Oncol. 2021. PMID: 34591419
-
Combined Treatment of Uterine Leiomyosarcoma with Gamma Secretase Inhibitor MK-0752 and Chemotherapeutic Agents Decreases Cellular Invasion and Increases Apoptosis.Cancers (Basel). 2024 Jun 10;16(12):2184. doi: 10.3390/cancers16122184. Cancers (Basel). 2024. PMID: 38927890 Free PMC article.
-
Surgical and oncologic outcomes of hyperthermic intraperitoneal chemotherapy for uterine leiomyosarcoma: A systematic review of literature.Gynecol Oncol. 2021 Apr;161(1):70-77. doi: 10.1016/j.ygyno.2020.12.032. Epub 2021 Jan 6. Gynecol Oncol. 2021. PMID: 33419612
Cited by
-
Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1.Cancers (Basel). 2022 Sep 12;14(18):4424. doi: 10.3390/cancers14184424. Cancers (Basel). 2022. PMID: 36139584 Free PMC article.
-
Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects.Biomolecules. 2024 Mar 23;14(4):387. doi: 10.3390/biom14040387. Biomolecules. 2024. PMID: 38672405 Free PMC article. Review.
-
Chemical Composition and Biological Activities of the Leaf Essential Oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia.Antibiotics (Basel). 2022 Nov 3;11(11):1547. doi: 10.3390/antibiotics11111547. Antibiotics (Basel). 2022. PMID: 36358202 Free PMC article.
-
Curdione combined with borneol treats bacterial mixed HPV infection by regulating the crosstalk among immune cells.Front Immunol. 2025 Jan 22;16:1503355. doi: 10.3389/fimmu.2025.1503355. eCollection 2025. Front Immunol. 2025. PMID: 39911394 Free PMC article.
-
Cytotoxicity evaluation of Curcuma aromatica Salisb. rhizome extract via apoptosis and reactive oxygen species generation in human gastric cancer cells.3 Biotech. 2025 Jun;15(6):153. doi: 10.1007/s13205-025-04318-1. Epub 2025 May 6. 3 Biotech. 2025. PMID: 40342537
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

