Near-Infrared Fluorescence Imaging and Photodynamic Therapy for Liver Tumors
- PMID: 33718233
- PMCID: PMC7947679
- DOI: 10.3389/fonc.2021.638327
Near-Infrared Fluorescence Imaging and Photodynamic Therapy for Liver Tumors
Abstract
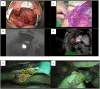
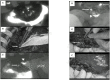
Surgery with fluorescence equipment has improved to treat the malignant viscera, including hepatobiliary and pancreatic neoplasms. In both open and minimally invasive surgeries, optical imaging using near-infrared (NIR) fluorescence is used to assess anatomy and function in real time. Here, we review a variety of publications related to clinical applications of NIR fluorescence imaging in liver surgery. We have developed a novel nanoparticle (indocyanine green lactosome) that is biocompatible and can be used for imaging cancer tissues and also as a drug delivery system. To date, stable particles are formed in blood and have an ~10-20 h half-life. Particles labeled with a NIR fluorescent agent have been applied to cancer tissues by the enhanced permeability and retention effect in animals. Furthermore, this article reviews recent developments in photodynamic therapy with NIR fluorescence imaging, which may contribute and accelerate the innovative treatments for liver tumors.
Keywords: indocyanine green lactosome; liver surgery navigation; liver tumors; near-infrared fluorescence imaging; photodynamic therapy.
Copyright © 2021 Kaibori, Kosaka, Matsui, Ishizaki, Matsushima, Tsuda, Hishikawa, Okumura and Sekimoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Rubens FD, Ruel M, Fremes SE. A new and simplified method for coronary and graft imaging during CABG. Heart Surg Forum. (2002) 5:141–4. - PubMed
-
- Taggart DP, Choudhary B, Anastasiadis K, Abu-Omar Y, Balacumaraswani L, Pigott DW. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg. (2003) 75:870–3. 10.1016/S0003-4975(02)04669-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

