Amentoflavone as an Ally in the Treatment of Cutaneous Leishmaniasis: Analysis of Its Antioxidant/Prooxidant Mechanisms
- PMID: 33718267
- PMCID: PMC7950538
- DOI: 10.3389/fcimb.2021.615814
Amentoflavone as an Ally in the Treatment of Cutaneous Leishmaniasis: Analysis of Its Antioxidant/Prooxidant Mechanisms
Abstract
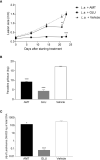
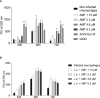
Treatment of leishmaniasis is a challenging subject. Although available, chemotherapy is limited, presenting toxicity and adverse effects. New drugs with antileishmanial activity are being investigated, such as antiparasitic compounds derived from plants. In this work, we investigated the antileishmanial activity of the biflavonoid amentoflavone on the protozoan Leishmania amazonensis. Although the antileishmanial activity of amentoflavone has already been reported in vitro, the mechanisms involved in the parasite death, as well as its action in vivo, remain unknown. Amentoflavone demonstrated activity on intracellular amastigotes in macrophages obtained from BALB/c mice (IC50 2.3 ± 0.93 μM). No cytotoxicity was observed and the selectivity index was estimated as greater than 10. Using BALB/c mice infected with L. amazonensis we verified the effect of an intralesional treatment with amentoflavone (0.05 mg/kg/dose, in a total of 5 doses every 4 days). Parasite quantification demonstrated that amentoflavone reduced the parasite load in treated footpads (46.3% reduction by limiting dilution assay and 56.5% reduction by Real Time Polymerase Chain Reaction). Amentoflavone decreased the nitric oxide production in peritoneal macrophages obtained from treated animals. The treatment also increased the expression of ferritin and decreased iNOS expression at the site of infection. Furthemore, it increased the production of ROS in peritoneal macrophages infected in vitro. The increase of ROS in vitro, associated with the reduction of NO and iNOS expression in vivo, points to the antioxidant/prooxidant potential of amentoflavone, which may play an important role in the balance between inflammatory and anti-inflammatory patterns at the infection site. Taken together these results suggest that amentoflavone has the potential to be used in the treatment of cutaneous leishmaniasis, working as an ally in the control and development of the lesion.
Keywords: Glucantime; amentoflavone; antileishmanial activity; biflavonoid; cutaneous leishmaniasis; intralesional treatment; prooxidant.
Copyright © 2021 Rizk, Santos-Pereira, Gervazoni, Hardoim, Cardoso, de Souza, Pelajo-Machado, Carollo, de Arruda, Almeida-Amaral, Zaverucha-do-Valle and Calabrese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Amentoflavone isolated from Selaginella sellowii Hieron induces mitochondrial dysfunction in Leishmania amazonensis promastigotes.Parasitol Int. 2022 Feb;86:102458. doi: 10.1016/j.parint.2021.102458. Epub 2021 Sep 9. Parasitol Int. 2022. PMID: 34509671
-
In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis.Mem Inst Oswaldo Cruz. 2014 Dec;109(8):1050-6. doi: 10.1590/0074-0276140312. Mem Inst Oswaldo Cruz. 2014. PMID: 25591109 Free PMC article.
-
Evaluation of total phenolic fraction derived from extra virgin olive oil for its antileishmanial activity.Phytomedicine. 2018 Aug 1;47:143-150. doi: 10.1016/j.phymed.2018.04.030. Epub 2018 May 10. Phytomedicine. 2018. PMID: 30166099
-
Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c.Nitric Oxide. 2016 Aug 31;58:51-8. doi: 10.1016/j.niox.2016.06.004. Epub 2016 Jun 18. Nitric Oxide. 2016. PMID: 27328771 Review.
-
Towards effective cutaneous leishmaniasis treatment with light-based technologies. A systematic review and meta-analysis of preclinical studies.J Photochem Photobiol B. 2021 Aug;221:112236. doi: 10.1016/j.jphotobiol.2021.112236. Epub 2021 May 31. J Photochem Photobiol B. 2021. PMID: 34090038
Cited by
-
Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii.Int J Mol Sci. 2022 Dec 23;24(1):229. doi: 10.3390/ijms24010229. Int J Mol Sci. 2022. PMID: 36613672 Free PMC article.
-
In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter pylori.Molecules. 2021 Dec 21;27(1):20. doi: 10.3390/molecules27010020. Molecules. 2021. PMID: 35011255 Free PMC article.
-
Comparative evaluation of silver nanoparticles and human platelet rich-plasma versus traditional therapy in the treatment of murine chronic toxoplasmosis.J Parasit Dis. 2024 Jun;48(2):217-228. doi: 10.1007/s12639-023-01642-2. Epub 2024 Mar 1. J Parasit Dis. 2024. PMID: 38840885 Free PMC article.
-
Chemopreventive mechanisms of amentoflavone: recent trends and advancements.Naunyn Schmiedebergs Arch Pharmacol. 2023 May;396(5):865-876. doi: 10.1007/s00210-023-02416-6. Epub 2023 Feb 11. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36773053 Review.
-
Tissue-Specific Profiling of Biflavonoids in Ginkgo (Ginkgo biloba L.).Plants (Basel). 2022 Dec 28;12(1):147. doi: 10.3390/plants12010147. Plants (Basel). 2022. PMID: 36616276 Free PMC article.
References
-
- Brasil (2017). “Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis,” in Manual de vigilância da leishmaniose tegumentar (Brasília: Ministério da Saúde; ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

