Proto-Oncogenes and Cell Cycle Gene Expression in Normal and Neoplastic Oral Epithelial Cells Stimulated With Soluble Factors From Single and Dual Biofilms of Candida albicans and Staphylococcus aureus
- PMID: 33718274
- PMCID: PMC7947338
- DOI: 10.3389/fcimb.2021.627043
Proto-Oncogenes and Cell Cycle Gene Expression in Normal and Neoplastic Oral Epithelial Cells Stimulated With Soluble Factors From Single and Dual Biofilms of Candida albicans and Staphylococcus aureus
Abstract
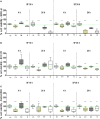
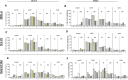
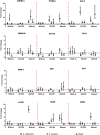
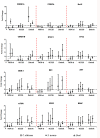
This study was aimed at analyzing proto-oncogenic signaling pathway activation in normal oral keratinocytes (NOK-si) and neoplastic cell lines (SCC 25 and Detroit 562) stimulated with metabolites (soluble factors) from single and dual biofilms of Candida albicans and Staphylococcus aureus. Soluble factors (SF) from early (16-h) and mature (36-h) biofilms of C. albicans and S. aureus were collected and incubated with cell cultures, which were subsequently evaluated using gene expression via RT-qPCR, cell viability via AlamarBlueTM, and flow cytometry cell cycle analysis. In general, exposure to the SF of early and mature biofilms from C. albicans and dual species caused a major reduction in NOK-si cell viability and enhanced the sub G0 phase. This led to a decrease in gene expression. However, in this cell line, SF of S. aureus biofilms upregulated the CDKN1A gene followed by the maintenance of cell viability and a significant increase in the G2/M population. For tumor cells, SCC 25 and Detroit 562, the stimuli of SF biofilms upregulated oncogenes such as hRAS and mTOR, as well as Bcl-2 and CDKN1A. SCC 25 and Detroit 562 cells could survive even after 24 h of stimuli from both SF (early and mature). This occurred without significant changes taking place in the cell cycle progression for SCC 25, and with a significant tendency to increase the G2/M phase for Detroit 562. These results point to the fact that metabolites from prevalent clinical fungal and bacterial biofilms, C. albicans and S. aureus, can disrupt the homeostasis of normal and neoplastic oral epithelial cells. This changes proto-oncogenes' expression, specifically PI3KCA, hRAS, mTOR, BRAF, and cell cycle genes CDKN1A and Bcl-2, thus causing a disturbance in cell viability, survival, and the cell cycle profile.
Keywords: Candida albicans; Staphylococcus aureus; biofilms; gene expression; metabolites; oral cancer.
Copyright © 2021 Amaya Arbeláez, de Paula e Silva, Navegante, Valente, Barbugli and Vergani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Baena-Monroy T., Moreno-Maldonado V., Franco-Martínez F., Aldape-Barrios B., Quindós G., Sánchez-Vargas L. O. (2005). Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral. Patol. Oral. Cir. Bucal 1, E27–E39. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

