Theileria annulata Subtelomere-Encoded Variable Secreted Protein-TA05575 Binds to Bovine RBMX2
- PMID: 33718289
- PMCID: PMC7952517
- DOI: 10.3389/fcimb.2021.644983
Theileria annulata Subtelomere-Encoded Variable Secreted Protein-TA05575 Binds to Bovine RBMX2
Abstract
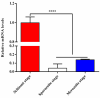
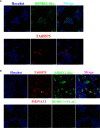
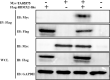
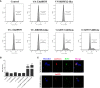
Tropical theileriosis is the disease caused by tick-transmitted apicomplexan parasite Theileria annulata, which has ability to transform bovine leukocytes, including B cells, macrophage cells, and dendritic cells. The T. annulata transformed cells are characterized as uncontrolled proliferation and shared some cancer-like phenotypes. The mechanism of the transformation by T. annulata is still not understood well. In previous reports, the subtelomere-encoded variable secreted proteins (SVSP) of T. parva were considered to contribute to phenotypic changes of the host cell, but the role of SVSP of T. annulata in host-pathogen relationship remains unknown. In the present study, a member of SVSP family, TA05575 of T. annulata was selected as the target molecule to analyze its expression profiles in different life cycle stages of T. annulata by qPCR and investigate its subcellular distribution of different passages of T. annulata transformed cells using confocal experiments. From the results, the transcription level of TA05575 at schizont stage was significantly higher than the other two life stages of T. annulata, and the protein of TA05575 was mainly distributed in nucleus of T. annulata infected cells. In addition, the potential proteins of host cells interacting with TA05575 were screened by Yeast-two hybrid system. The results of Co-IP experiment confirmed that TA05575 interacted with RBMX2-like protein that participated in transcription regulation of cells. In addition, a novel BiFC assay and flow cytometry were carried out, and the results further revealed that TA05575-RBMX2-like pair was directly interacted in cell context. Moreover, this interacting pair was found to distribute in intracellular compartments of HEK293T cells by using confocal microscopy. The results of the present study suggest that TA05575 may contribute for cells transformation due its distribution. According to the function of RBMX2, the interaction of TA05575 and RMMX2-like will provide a new information to further understand the mechanisms of cells transformation by T. annulata.
Keywords: BiFC; Co-IP; RBMX2-like; TA05575; Theileria annulata; Yeast-two hybrid.
Copyright © 2021 Li, Liu, Zhao, Ma, Liu, Li, Guan, Luo and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Theileria annulata subtelomere-encoded variable secreted protein-TA05560 interacts with bovine RNA binding motif protein 39 (RBM39).Acta Trop. 2024 Apr;252:107133. doi: 10.1016/j.actatropica.2024.107133. Epub 2024 Jan 26. Acta Trop. 2024. PMID: 38280638
-
Screening and identification of Theileria annulata subtelomere-encoded variable secreted protein-950454 (SVSP454) interacting proteins from bovine B cells.Parasit Vectors. 2021 Jun 11;14(1):319. doi: 10.1186/s13071-021-04820-4. Parasit Vectors. 2021. PMID: 34116718 Free PMC article.
-
Theileria annulata SVSP455 interacts with host HSP60.Parasit Vectors. 2022 Aug 30;15(1):308. doi: 10.1186/s13071-022-05427-z. Parasit Vectors. 2022. PMID: 36042502 Free PMC article.
-
Immunity and vaccine development in the bovine theilerioses.Adv Parasitol. 1999;44:41-97. doi: 10.1016/s0065-308x(08)60230-4. Adv Parasitol. 1999. PMID: 10563395 Review.
-
Transformation of leukocytes by Theileria parva and T. annulata.Annu Rev Microbiol. 1999;53:1-42. doi: 10.1146/annurev.micro.53.1.1. Annu Rev Microbiol. 1999. PMID: 10547684 Review.
Cited by
-
The Tick-Borne Pathogens: An Overview of China's Situation.Acta Parasitol. 2023 Mar;68(1):1-20. doi: 10.1007/s11686-023-00658-1. Epub 2023 Jan 16. Acta Parasitol. 2023. PMID: 36642777 Free PMC article. Review.
-
Expression profile of microRNAs in bovine lymphocytes infected with Theileria annulata and treated with buparvaquone.Parasitol Res. 2024 Sep 9;123(9):318. doi: 10.1007/s00436-024-08341-8. Parasitol Res. 2024. PMID: 39249568
References
-
- Ahmadi R. D., Sharifi T. M., Alikhani M., Parsamatin P., Sahraneshin S. F., Sabbaghian M., et al. . (2015). Isoform-Level Gene Expression Profiles of Human Y Chromosome Azoospermia Factor Genes and Their X Chromosome Paralogs in the Testicular Tissue of Non-Obstructive Azoospermia Patients. J. Proteome Res. 14 (9), 3595–3605. 10.1021/acs.jproteome.5b00520 - DOI - PubMed
-
- Al-Deeb M. A., Muza_ar S. B., Abu-Zeid Y. A., Enan M. R., Karim S. (2015). First Record of a Spotted Fever Group Rickettsia sp. and Theileria annulata in Hyalomma dromedarii (Acari: Ixodidae) Ticks in the United Arab Emirates. Fla Entomol. 98, 135–139. 10.1653/024.098.0123 - DOI
-
- Anupama R., Srinivasan S. R., Parthiban M. (2015). Molecular studies on theileriosis and identification of Theileria orientalis in India using PCR. Indian Vet. J. 92, 9–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

