Hypertrophic Cardiomyopathy in Children: Pathophysiology, Diagnosis, and Treatment of Non-sarcomeric Causes
- PMID: 33718303
- PMCID: PMC7947260
- DOI: 10.3389/fped.2021.632293
Hypertrophic Cardiomyopathy in Children: Pathophysiology, Diagnosis, and Treatment of Non-sarcomeric Causes
Abstract
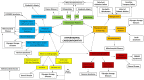
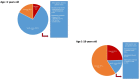
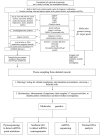
Hypertrophic cardiomyopathy (HCM) is a myocardial disease characterized by left ventricular hypertrophy not solely explained by abnormal loading conditions. Despite its rare prevalence in pediatric age, HCM carries a relevant risk of mortality and morbidity in both infants and children. Pediatric HCM is a large heterogeneous group of disorders. Other than mutations in sarcomeric genes, which represent the most important cause of HCM in adults, childhood HCM includes a high prevalence of non-sarcomeric causes, including inherited errors of metabolism (i.e., glycogen storage diseases, lysosomal storage diseases, and fatty acid oxidation disorders), malformation syndromes, neuromuscular diseases, and mitochondrial disease, which globally represent up to 35% of children with HCM. The age of presentation and the underlying etiology significantly impact the prognosis of children with HCM. Moreover, in recent years, different targeted approaches for non-sarcomeric etiologies of HCM have emerged. Therefore, the etiological diagnosis is a fundamental step in designing specific management and therapy in these subjects. The present review aims to provide an overview of the non-sarcomeric causes of HCM in children, focusing on the pathophysiology, clinical features, diagnosis, and treatment of these rare disorders.
Keywords: children; diagnosis; etiology; hypertrophic cardiomyopathy; treatment.
Copyright © 2021 Monda, Rubino, Lioncino, Di Fraia, Pacileo, Verrillo, Cirillo, Caiazza, Fusco, Esposito, Fimiani, Palmiero, Pacileo, Calabrò, Russo and Limongelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Targeted Therapies in Pediatric and Adult Patients With Hypertrophic Heart Disease: From Molecular Pathophysiology to Personalized Medicine.Circ Heart Fail. 2023 Aug;16(8):e010687. doi: 10.1161/CIRCHEARTFAILURE.123.010687. Epub 2023 Jul 21. Circ Heart Fail. 2023. PMID: 37477018 Review.
-
Myocardial deformation abnormalities in pediatric hypertrophic cardiomyopathy: are all etiologies identical?Eur J Echocardiogr. 2008 Nov;9(6):784-90. doi: 10.1093/ejechocard/jen150. Epub 2008 Apr 27. Eur J Echocardiogr. 2008. PMID: 18490283
-
Echocardiographic characteristics of PRKAG2 syndrome: a research using three-dimensional speckle tracking echocardiography compared with sarcomeric hypertrophic cardiomyopathy.Cardiovasc Ultrasound. 2022 May 5;20(1):14. doi: 10.1186/s12947-022-00284-3. Cardiovasc Ultrasound. 2022. PMID: 35509080 Free PMC article.
-
Mimics of Hypertrophic Cardiomyopathy - Diagnostic Clues to Aid Early Identification of Phenocopies.Arrhythm Electrophysiol Rev. 2013 Apr;2(1):36-40. doi: 10.15420/aer.2013.2.1.36. Arrhythm Electrophysiol Rev. 2013. PMID: 26835038 Free PMC article.
-
Common presentation of rare diseases: Left ventricular hypertrophy and diastolic dysfunction.Int J Cardiol. 2018 Apr 15;257:344-350. doi: 10.1016/j.ijcard.2018.01.006. Int J Cardiol. 2018. PMID: 29506729 Review.
Cited by
-
The Beneficial Atrial Septal Defect Shunt in Hypertrophic Cardiomyopathy-When Closure Is Not the Answer.J Soc Cardiovasc Angiogr Interv. 2024 Sep 2;3(10):102281. doi: 10.1016/j.jscai.2024.102281. eCollection 2024 Oct. J Soc Cardiovasc Angiogr Interv. 2024. PMID: 39525996 Free PMC article. No abstract available.
-
Clinical and Genetic Screening for Hypertrophic Cardiomyopathy in Paediatric Relatives: Changing Paradigms in Clinical Practice.J Clin Med. 2023 Apr 9;12(8):2788. doi: 10.3390/jcm12082788. J Clin Med. 2023. PMID: 37109125 Free PMC article. Review.
-
Brazilian Guideline for Exercise Testing in Children and Adolescents - 2024.Arq Bras Cardiol. 2024 Sep 16;121(8):e20240525. doi: 10.36660/abc.20240525. Arq Bras Cardiol. 2024. PMID: 39292116 Free PMC article. English, Portuguese.
-
Patterns of Left Ventricular Remodelling in Children and Young Patients with Hypertrophic Cardiomyopathy.J Clin Med. 2024 Jul 4;13(13):3937. doi: 10.3390/jcm13133937. J Clin Med. 2024. PMID: 38999502 Free PMC article.
-
Hypertrophic Cardiomyopathy in RASopathies: Diagnosis, Clinical Characteristics, Prognostic Implications, and Management.Heart Fail Clin. 2022 Jan;18(1):19-29. doi: 10.1016/j.hfc.2021.07.004. Epub 2021 Oct 25. Heart Fail Clin. 2022. PMID: 34776080 Free PMC article. Review.
References
-
- Authors/Task Force members. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. . 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. (2014). 35:2733–79. 10.1093/eurheartj/ehu284 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

