Ca2+ Complexation With Relevant Bioligands in Aqueous Solution: A Speciation Study With Implications for Biological Fluids
- PMID: 33718329
- PMCID: PMC7953420
- DOI: 10.3389/fchem.2021.640219
Ca2+ Complexation With Relevant Bioligands in Aqueous Solution: A Speciation Study With Implications for Biological Fluids
Abstract
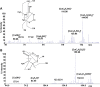
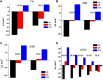
A speciation study on the interaction between Ca2+ and ligands of biological interest in aqueous solution is reported. The ligands under study are l-cysteine (Cys), d-penicillamine (PSH), reduced glutathione (GSH), and oxidized glutathione (GSSG). From the elaboration of the potentiometric experimental data the most likely speciation patterns obtained are characterized by only protonated species with a 1:1 metal to ligand ratio. In detail, two species, CaLH2 and CaLH, for systems containing Cys, PSH, and GSH, and five species, CaLH5, CaLH4, CaLH3, CaLH2, and CaLH, for system containing GSSG, were observed. The potentiometric titrations were performed at different temperatures (15 ≤ t/°C ≤ 37, at I = 0.15 mol L-1). The enthalpy and entropy change values were calculated for all systems, and the dependence of the formation constants of the complex species on the temperature was evaluated. 1H NMR spectroscopy, MALDI mass spectrometry, and tandem mass spectrometry (MS/MS) investigations on Ca2+-ligand solutions were also employed, confirming the interactions and underlining characteristic complexing behaviors of Cys, PSH, GSH, and GSSG toward Ca2+. The results of the analysis of 1H NMR experimental data are in full agreement with potentiometric ones in terms of speciation models and stability constants of the species. MALDI mass spectrometry and tandem mass spectrometry (MS/MS) analyses confirm the formation of Ca2+-L complex species and elucidate the mechanism of interaction. On the basis of speciation models, simulations of species formation under conditions of some biological fluids were reported. The sequestering ability of Cys, PSH, GSH, and GSSG toward Ca2+ was evaluated under different conditions of pH and temperature and under physiological condition.
Keywords: 1H NMR spectroscopy; Ca2+; biological ligands; mass spectrometry; potentiometry; sequestration; speciation in biological fluids; thermodynamic parameters.
Copyright © 2021 Aiello, Carnamucio, Cordaro, Foti, Napoli and Giuffrè.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Aiello D., Cardiano P., Cigala R. M., Gans P., Giacobello F., Giuffrè O., et al. (2017). Sequestering ability of oligophosphate ligands toward Al3+ in aqueous solution. J. Chem. Eng. Data 62, 3981–3990. 10.1021/acs.jced.7b00685 - DOI
-
- Aiello D., Furia E., Siciliano C., Bongiorno D., Napoli A. (2018a). Study of the coordination of ortho-tyrosine and trans-4-hydroxyproline with aluminum(III) and iron(III). J. Mol. Liq 269, 387–397. 10.1016/j.molliq.2018.08.074 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

