Deletion of Mea6 in Cerebellar Granule Cells Impairs Synaptic Development and Motor Performance
- PMID: 33718348
- PMCID: PMC7946997
- DOI: 10.3389/fcell.2020.627146
Deletion of Mea6 in Cerebellar Granule Cells Impairs Synaptic Development and Motor Performance
Abstract
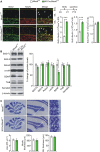
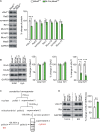
The cerebellum is conceptualized as a processor of complex movements. Many diseases with gene-targeted mutations, including Fahr's disease associated with the loss-of-function mutation of meningioma expressed antigen 6 (Mea6), exhibit cerebellar malformations, and abnormal motor behaviors. We previously reported that the defects in cerebellar development and motor performance of Nestin-Cre;Mea6 F/F mice are severer than those of Purkinje cell-targeted pCP2-Cre;Mea6 F/F mice, suggesting that Mea6 acts on other types of cerebellar cells. Hence, we investigated the function of Mea6 in cerebellar granule cells. We found that mutant mice with the specific deletion of Mea6 in granule cells displayed abnormal posture, balance, and motor learning, as indicated in footprint, head inclination, balanced beam, and rotarod tests. We further showed that Math1-Cre;Mea6 F/F mice exhibited disrupted migration of granule cell progenitors and damaged parallel fiber-Purkinje cell synapses, which may be related to impaired intracellular transport of vesicular glutamate transporter 1 and brain-derived neurotrophic factor. The present findings extend our previous work and may help to better understand the pathogenesis of Fahr's disease.
Keywords: Fahr’s syndrome; Mea6; granule cell; malformation; motor performance; vGluT1.
Copyright © 2021 Wang, Zhou, Cai, Xu, Xu, Li and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
MEA6 Deficiency Impairs Cerebellar Development and Motor Performance by Tethering Protein Trafficking.Front Cell Neurosci. 2019 Jun 11;13:250. doi: 10.3389/fncel.2019.00250. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31244610 Free PMC article.
-
cTAGE5/MEA6 plays a critical role in neuronal cellular components trafficking and brain development.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):E9449-E9458. doi: 10.1073/pnas.1804083115. Epub 2018 Sep 17. Proc Natl Acad Sci U S A. 2018. PMID: 30224460 Free PMC article.
-
The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice.J Neurosci. 2001 Jan 1;21(1):10-7. doi: 10.1523/JNEUROSCI.21-01-00010.2001. J Neurosci. 2001. PMID: 11150314 Free PMC article.
-
Maturation of Purkinje cell firing properties relies on neurogenesis of excitatory neurons.Elife. 2021 Sep 20;10:e68045. doi: 10.7554/eLife.68045. Elife. 2021. PMID: 34542409 Free PMC article.
-
Glutamate-receptor-like molecule GluRδ2 involved in synapse formation at parallel fiber-Purkinje neuron synapses.Cerebellum. 2012 Mar;11(1):71-7. doi: 10.1007/s12311-010-0170-0. Cerebellum. 2012. PMID: 20387025 Review.
Cited by
-
Genome-wide association study of haploid female fertility (HFF) and haploid male fertility (HMF) in BS39-derived doubled haploid maize lines.Theor Appl Genet. 2024 Dec 11;138(1):5. doi: 10.1007/s00122-024-04789-5. Theor Appl Genet. 2024. PMID: 39663254 Review.
-
Regulation of cerebellar network development by granule cells and their molecules.Front Mol Neurosci. 2023 Jul 14;16:1236015. doi: 10.3389/fnmol.2023.1236015. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37520428 Free PMC article. Review.
-
Ablation of Mea6/cTAGE5 in oligodendrocytes significantly impairs white matter structure and lipid content.Life Metab. 2023 Mar 23;2(2):load010. doi: 10.1093/lifemeta/load010. eCollection 2023 Apr. Life Metab. 2023. PMID: 39872732 Free PMC article.
-
G protein-coupled receptors: pivotal hubs in gastric cancer malignancy-from multidimensional crosstalk to precision therapeutics.J Transl Med. 2025 Aug 7;23(1):879. doi: 10.1186/s12967-025-06851-2. J Transl Med. 2025. PMID: 40775711 Free PMC article. Review.
-
Sepsis Impairs Purkinje Cell Functions and Motor Behaviors Through Microglia Activation.Cerebellum. 2024 Apr;23(2):329-339. doi: 10.1007/s12311-023-01531-7. Epub 2023 Feb 15. Cerebellum. 2024. PMID: 36790600
References
-
- Borghesani P. R., Peyrin J. M., Klein R., Rubin J., Carter A. R., Schwartz P. M., et al. (2002). BDNF stimulates migration of cerebellar granule cells. Development 129 1435–1442. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

