Clathrin Heavy Chain 1 Plays Essential Roles During Oocyte Meiotic Spindle Formation and Early Embryonic Development in Sheep
- PMID: 33718352
- PMCID: PMC7946971
- DOI: 10.3389/fcell.2021.609311
Clathrin Heavy Chain 1 Plays Essential Roles During Oocyte Meiotic Spindle Formation and Early Embryonic Development in Sheep
Abstract
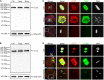
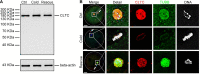
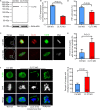
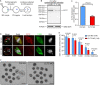
As a major protein of the polyhedral coat of coated pits and vesicles, clathrin molecules have been shown to play a stabilization role for kinetochore fibers of the mitotic spindle by acting as inter-microtubule bridges. Clathrin heavy chain 1 (CLTC), the basic subunit of the clathrin coat, plays vital roles in both spindle assembly and chromosome congression during somatic-cell mitosis. However, its function in oocyte meiotic maturation and early embryo development in mammals, especially in domesticated animals, has not been fully investigated. In this study, the expression profiles and functional roles of CLTC in sheep oocytes were investigated. Our results showed that the expression of CLTC was maintained at a high level from the germinal vesicle (GV) stage to metaphase II stage and that CLTC was distributed diffusely in the cytoplasm of cells at interphase, from the GV stage to the blastocyst stage. After GV breakdown (GVBD), CLTC co-localized with beta-tubulin during metaphase. Oocyte treatments with taxol, nocodazole, or cold did not affect CLTC expression levels but led to disorders of its distribution. Functional impairment of CLTC by specific morpholino injections in GV-stage oocytes led to disruptions in spindle assembly and chromosomal alignment, accompanied by impaired first polar body (PB1) emissions. In addition, knockdown of CLTC before parthenogenetic activation disrupted spindle formation and impaired early embryo development. Taken together, the results demonstrate that CLTC plays a vital role in sheep oocyte maturation via the regulation of spindle dynamics and an essential role during early embryo development.
Keywords: CLTC; chromosome congression; early embryo development; oocyte; spindle assembly.
Copyright © 2021 Han, Hao, Zhou, Wang, Wen, Wang, Zhang and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cotterill M., Harris S. E., Collado Fernandez E., Lu J., Huntriss J. D., Campbell B. K., et al. (2013). The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol. Hum. Reprod. 19 444–450. 10.1093/molehr/gat013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

