Perspectives on Organelle Interaction, Protein Dysregulation, and Cancer Disease
- PMID: 33718356
- PMCID: PMC7946981
- DOI: 10.3389/fcell.2021.613336
Perspectives on Organelle Interaction, Protein Dysregulation, and Cancer Disease
Abstract
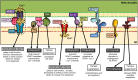
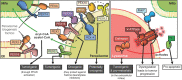
In recent decades, compelling evidence has emerged showing that organelles are not static structures but rather form a highly dynamic cellular network and exchange information through membrane contact sites. Although high-throughput techniques facilitate identification of novel contact sites (e.g., organelle-organelle and organelle-vesicle interactions), little is known about their impact on cellular physiology. Moreover, even less is known about how the dysregulation of these structures impacts on cellular function and therefore, disease. Particularly, cancer cells display altered signaling pathways involving several cell organelles; however, the relevance of interorganelle communication in oncogenesis and/or cancer progression remains largely unknown. This review will focus on organelle contacts relevant to cancer pathogenesis. We will highlight specific proteins and protein families residing in these organelle-interfaces that are known to be involved in cancer-related processes. First, we will review the relevance of endoplasmic reticulum (ER)-mitochondria interactions. This section will focus on mitochondria-associated membranes (MAMs) and particularly the tethering proteins at the ER-mitochondria interphase, as well as their role in cancer disease progression. Subsequently, the role of Ca2+ at the ER-mitochondria interphase in cancer disease progression will be discussed. Members of the Bcl-2 protein family, key regulators of cell death, also modulate Ca2+ transport pathways at the ER-mitochondria interphase. Furthermore, we will review the role of ER-mitochondria communication in the regulation of proteostasis, focusing on the ER stress sensor PERK (PRKR-like ER kinase), which exerts dual roles in cancer. Second, we will review the relevance of ER and mitochondria interactions with other organelles. This section will focus on peroxisome and lysosome organelle interactions and their impact on cancer disease progression. In this context, the peroxisome biogenesis factor (PEX) gene family has been linked to cancer. Moreover, the autophagy-lysosome system is emerging as a driving force in the progression of numerous human cancers. Thus, we will summarize our current understanding of the role of each of these organelles and their communication, highlighting how alterations in organelle interfaces participate in cancer development and progression. A better understanding of specific organelle communication sites and their relevant proteins may help to identify potential pharmacological targets for novel therapies in cancer control.
Keywords: cancer; endoplasmic reticulum; interorganelle communication; lysosome; mitochondria; peroxisome.
Copyright © 2021 Díaz, Sandoval-Bórquez, Bravo-Sagua, Quest and Lavandero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Differential Effect of Glucose on ER-Mitochondria Ca2+ Exchange Participates in Insulin Secretion and Glucotoxicity-Mediated Dysfunction of β-Cells.Diabetes. 2019 Sep;68(9):1778-1794. doi: 10.2337/db18-1112. Epub 2019 Jun 7. Diabetes. 2019. PMID: 31175102
-
Organelle crosstalk in the kidney.Kidney Int. 2019 Jun;95(6):1318-1325. doi: 10.1016/j.kint.2018.11.035. Epub 2019 Mar 4. Kidney Int. 2019. PMID: 30878214 Review.
-
Mitochondria-Associated Membranes and ER Stress.Curr Top Microbiol Immunol. 2018;414:73-102. doi: 10.1007/82_2017_2. Curr Top Microbiol Immunol. 2018. PMID: 28349285 Review.
-
ER-organelle contacts: A signaling hub for neurological diseases.Pharmacol Res. 2024 May;203:107149. doi: 10.1016/j.phrs.2024.107149. Epub 2024 Mar 20. Pharmacol Res. 2024. PMID: 38518830 Review.
-
Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role.Cell Calcium. 2021 Mar;94:102343. doi: 10.1016/j.ceca.2020.102343. Epub 2021 Jan 2. Cell Calcium. 2021. PMID: 33418313 Review.
Cited by
-
Current Methods for Identifying Plasma Membrane Proteins as Cancer Biomarkers.Membranes (Basel). 2023 Apr 5;13(4):409. doi: 10.3390/membranes13040409. Membranes (Basel). 2023. PMID: 37103836 Free PMC article. Review.
-
The Role of ER Stress and the Unfolded Protein Response in Cancer.Cancer Genomics Proteomics. 2025 May-Jun;22(3):363-381. doi: 10.21873/cgp.20507. Cancer Genomics Proteomics. 2025. PMID: 40280715 Free PMC article. Review.
-
Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer's Disease.Biology (Basel). 2024 Mar 14;13(3):185. doi: 10.3390/biology13030185. Biology (Basel). 2024. PMID: 38534454 Free PMC article. Review.
-
Key challenges and recommendations for defining organelle membrane contact sites.Nat Rev Mol Cell Biol. 2025 Jun 23. doi: 10.1038/s41580-025-00864-x. Online ahead of print. Nat Rev Mol Cell Biol. 2025. PMID: 40550870 Review.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process.Oxid Med Cell Longev. 2022 Aug 23;2022:7086807. doi: 10.1155/2022/7086807. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36052160 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

