SIRT6 Maintains Redox Homeostasis to Promote Porcine Oocyte Maturation
- PMID: 33718364
- PMCID: PMC7947247
- DOI: 10.3389/fcell.2021.625540
SIRT6 Maintains Redox Homeostasis to Promote Porcine Oocyte Maturation
Abstract
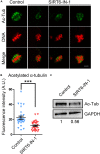
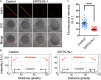
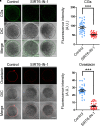
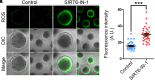
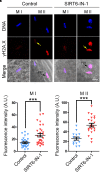
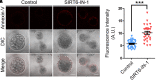
SIRT6, the sixth member of the sirtuin family proteins, has been characterized as a crucial regulator in multiple molecular pathways related to aging, including genome stability, DNA damage repair, telomere maintenance, and inflammation. However, the exact roles of SIRT6 during female germ cell development have not yet been fully determined. Here, we assessed the acquisition of meiotic competency of porcine oocytes by inhibition of SIRT6 activity. We observed that SIRT6 inhibition led to the oocyte meiotic defects by showing the impairment of polar body extrusion and cumulus cell expansion. Meanwhile, the compromised spindle/chromosome structure and actin dynamics were also present in SIRT6-inhibited oocytes. Moreover, SIRT6 inhibition resulted in the defective cytoplasmic maturation by displaying the disturbed distribution dynamics of cortical granules and their content ovastacin. Notably, we identified that transcript levels of genes related to oocyte meiosis, oxidative phosphorylation, and cellular senescence were remarkably altered in SIRT6-inhibited oocytes by transcriptome analysis and validated that the meiotic defects caused by SIRT6 inhibition might result from the excessive reactive oxygen species (ROS)-induced early apoptosis in oocytes. Taken together, our findings demonstrate that SIRT6 promotes the porcine oocyte meiotic maturation through maintaining the redox homeostasis.
Keywords: SIRT6; apoptosis; meiotic failure; oocyte maturation; redox homeostasis.
Copyright © 2021 Li, Miao, Chen and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

