Lysosomes and Cancer Progression: A Malignant Liaison
- PMID: 33718382
- PMCID: PMC7952443
- DOI: 10.3389/fcell.2021.642494
Lysosomes and Cancer Progression: A Malignant Liaison
Abstract
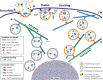
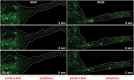
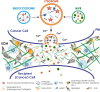
During primary tumorigenesis isolated cancer cells may undergo genetic or epigenetic changes that render them responsive to additional intrinsic or extrinsic cues, so that they enter a transitional state and eventually acquire an aggressive, metastatic phenotype. Among these changes is the alteration of the cell metabolic/catabolic machinery that creates the most permissive conditions for invasion, dissemination, and survival. The lysosomal system has emerged as a crucial player in this malignant transformation, making this system a potential therapeutic target in cancer. By virtue of their ubiquitous distribution in mammalian cells, their multifaced activities that control catabolic and anabolic processes, and their interplay with other organelles and the plasma membrane (PM), lysosomes function as platforms for inter- and intracellular communication. This is due to their capacity to adapt and sense nutrient availability, to spatially segregate specific functions depending on their position, to fuse with other compartments and with the PM, and to engage in membrane contact sites (MCS) with other organelles. Here we review the latest advances in our understanding of the role of the lysosomal system in cancer progression. We focus on how changes in lysosomal nutrient sensing, as well as lysosomal positioning, exocytosis, and fusion perturb the communication between tumor cells themselves and between tumor cells and their microenvironment. Finally, we describe the potential impact of MCS between lysosomes and other organelles in propelling cancer growth and spread.
Keywords: cancer progression; lysosomal exocytosis; lysosomal membrane contact sites; lysosome movement; lysosome positioning.
Copyright © 2021 Machado, Annunziata, van de Vlekkert, Grosveld and d’Azzo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Lysosomes in Cancer-At the Crossroad of Good and Evil.Cells. 2024 Mar 5;13(5):459. doi: 10.3390/cells13050459. Cells. 2024. PMID: 38474423 Free PMC article. Review.
-
Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells.PLoS Negl Trop Dis. 2012;6(3):e1583. doi: 10.1371/journal.pntd.0001583. Epub 2012 Mar 27. PLoS Negl Trop Dis. 2012. PMID: 22479662 Free PMC article.
-
Regulated lysosomal exocytosis mediates cancer progression.Sci Adv. 2015 Dec 18;1(11):e1500603. doi: 10.1126/sciadv.1500603. eCollection 2015 Dec. Sci Adv. 2015. PMID: 26824057 Free PMC article.
-
Lysosomal Biology and Function: Modern View of Cellular Debris Bin.Cells. 2020 May 4;9(5):1131. doi: 10.3390/cells9051131. Cells. 2020. PMID: 32375321 Free PMC article. Review.
-
Current methods to analyze lysosome morphology, positioning, motility and function.Traffic. 2022 May;23(5):238-269. doi: 10.1111/tra.12839. Epub 2022 Apr 24. Traffic. 2022. PMID: 35343629 Free PMC article. Review.
Cited by
-
Lysosomal positioning diseases: beyond substrate storage.Open Biol. 2022 Oct;12(10):220155. doi: 10.1098/rsob.220155. Epub 2022 Oct 26. Open Biol. 2022. PMID: 36285443 Free PMC article. Review.
-
4D Single-particle tracking with asynchronous read-out single-photon avalanche diode array detector.Nat Commun. 2024 Jul 23;15(1):6188. doi: 10.1038/s41467-024-50512-9. Nat Commun. 2024. PMID: 39043637 Free PMC article.
-
Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-κB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages.Int J Mol Sci. 2024 Mar 13;25(6):3246. doi: 10.3390/ijms25063246. Int J Mol Sci. 2024. PMID: 38542221 Free PMC article.
-
Disruption of Man-6-P-Dependent Sorting to Lysosomes Confers IGF1R-Mediated Apoptosis Resistance.Int J Mol Sci. 2025 Apr 10;26(8):3586. doi: 10.3390/ijms26083586. Int J Mol Sci. 2025. PMID: 40332073 Free PMC article.
-
Lysosomes in Cancer-At the Crossroad of Good and Evil.Cells. 2024 Mar 5;13(5):459. doi: 10.3390/cells13050459. Cells. 2024. PMID: 38474423 Free PMC article. Review.
References
-
- Agarwal A. K., Srinivasan N., Godbole R., More S. K., Budnar S., Gude R. P., et al. (2015). Role of tumor cell surface lysosome-associated membrane protein-1 (LAMP1) and its associated carbohydrates in lung metastasis. J. Cancer Res. Clin. Oncol. 141 1563–1574. 10.1007/s00432-015-1917-2 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

