Membrane Transport Proteins in Osteoclasts: The Ins and Outs
- PMID: 33718388
- PMCID: PMC7952445
- DOI: 10.3389/fcell.2021.644986
Membrane Transport Proteins in Osteoclasts: The Ins and Outs
Abstract
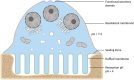
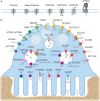
During bone resorption, the osteoclast must sustain an extraordinarily low pH environment, withstand immense ionic pressures, and coordinate nutrient and waste exchange across its membrane to sustain its unique structural and functional polarity. To achieve this, osteoclasts are equipped with an elaborate set of membrane transport proteins (pumps, transporters and channels) that serve as molecular 'gatekeepers' to regulate the bilateral exchange of ions, amino acids, metabolites and macromolecules across the ruffled border and basolateral domains. Whereas the importance of the vacuolar-ATPase proton pump and chloride voltage-gated channel 7 in osteoclasts has long been established, comparatively little is known about the contributions of other membrane transport proteins, including those categorized as secondary active transporters. In this Special Issue review, we provide a contemporary update on the 'ins and outs' of membrane transport proteins implicated in osteoclast differentiation, function and bone homeostasis and discuss their therapeutic potential for the treatment of metabolic bone diseases.
Keywords: CLCN7; V-ATPase; bone disease; ion channel; membrane transporter; osteoclast; osteoporosis; solute carrier.
Copyright © 2021 Ribet, Ng and Pavlos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Ion channels and transporters in osteoclasts.Arch Biochem Biophys. 2008 May 15;473(2):161-5. doi: 10.1016/j.abb.2008.03.029. Epub 2008 Mar 29. Arch Biochem Biophys. 2008. PMID: 18406337 Review.
-
Osteoclastic acid transport: mechanism and implications for physiological and pharmacological regulation.Miner Electrolyte Metab. 1994;20(1-2):31-9. Miner Electrolyte Metab. 1994. PMID: 8202050 Review.
-
Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice.J Bone Miner Res. 2008 Nov;23(11):1815-24. doi: 10.1359/jbmr.080613. J Bone Miner Res. 2008. PMID: 18597625
-
Cooperative electrogenic proton transport pathways in the plasma membrane of the proton-secreting osteoclast.Pflugers Arch. 2018 Jun;470(6):851-866. doi: 10.1007/s00424-018-2137-9. Epub 2018 Mar 17. Pflugers Arch. 2018. PMID: 29550927 Review.
-
The bisphosphonate tiludronate is a potent inhibitor of the osteoclast vacuolar H(+)-ATPase.J Bone Miner Res. 1996 Oct;11(10):1498-507. doi: 10.1002/jbmr.5650111017. J Bone Miner Res. 1996. PMID: 8889850
Cited by
-
Structure and function of the membrane microdomains in osteoclasts.Bone Res. 2023 Nov 21;11(1):61. doi: 10.1038/s41413-023-00294-5. Bone Res. 2023. PMID: 37989999 Free PMC article. Review.
-
Multiple Genetic Loci Associated with Pug Dog Thoracolumbar Myelopathy.Genes (Basel). 2023 Feb 1;14(2):385. doi: 10.3390/genes14020385. Genes (Basel). 2023. PMID: 36833311 Free PMC article.
-
Review of Cathepsin K Inhibitor Development and the Potential Role of Phytochemicals.Molecules. 2024 Dec 29;30(1):91. doi: 10.3390/molecules30010091. Molecules. 2024. PMID: 39795149 Free PMC article. Review.
-
Alterations in the microenvironment and the effects produced of TRPV5 in osteoporosis.J Transl Med. 2023 May 17;21(1):327. doi: 10.1186/s12967-023-04182-8. J Transl Med. 2023. PMID: 37198647 Free PMC article. Review.
-
Lysosomal biogenesis and function in osteoclasts: a comprehensive review.Front Cell Dev Biol. 2024 Aug 7;12:1431566. doi: 10.3389/fcell.2024.1431566. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39170917 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

