Maternal Lead Exposure Impairs Offspring Learning and Memory via Decreased GLUT4 Membrane Translocation
- PMID: 33718391
- PMCID: PMC7947239
- DOI: 10.3389/fcell.2021.648261
Maternal Lead Exposure Impairs Offspring Learning and Memory via Decreased GLUT4 Membrane Translocation
Abstract
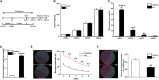
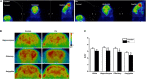
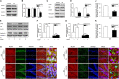
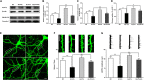
Lead (Pb) can cause a significant neurotoxicity in both adults and children, leading to the impairment to brain function. Pb exposure plays a key role in the impairment of learning and memory through synaptic neurotoxicity, resulting in the cognitive function. Researches have demonstrated that Pb exposure plays an important role in the etiology and pathogenesis of neurodegenerative diseases, such as Alzheimer's disease. However, the underlying mechanisms remain unclear. In the current study, a gestational Pb exposure (GLE) rat model was established to investigate the underlying mechanisms of Pb-induced cognitive impairment. We demonstrated that low-level gestational Pb exposure impaired spatial learning and memory as well as hippocampal synaptic plasticity at postnatal day 30 (PND 30) when the blood concentration of Pb had already recovered to normal levels. Pb exposure induced a decrease in hippocampal glucose metabolism by reducing glucose transporter 4 (GLUT4) levels in the cell membrane through the phosphatidylinositol 3 kinase-protein kinase B (PI3K-Akt) pathway. In vivo and in vitro GLUT4 over-expression increased the membrane translocation of GLUT4 and glucose uptake, and reversed the Pb-induced impairment to synaptic plasticity and cognition. These findings indicate that Pb exposure impairs synaptic plasticity by reducing the level of GLUT4 in the cell membrane as well as glucose uptake via the PI3K-Akt signaling pathway, demonstrating a novel mechanism for Pb exposure-induced neurotoxicity.
Keywords: PI3K-Akt; glucose transporter 4; hippocampus synaptic plasticity; lead; learning and memory.
Copyright © 2021 Zhao, Du, Wang, Wang, Cao, Chen, Song, Zheng and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The mechanism of lipopolysaccharide administration-induced cognitive function impairment caused by glucose metabolism disorder in adult rats.Saudi J Biol Sci. 2019 Sep;26(6):1268-1277. doi: 10.1016/j.sjbs.2019.06.017. Epub 2019 Jul 2. Saudi J Biol Sci. 2019. PMID: 31516357 Free PMC article.
-
[Relationships of glucose transporter 4 with cognitive changes induced by high fat diet and glucose metabolism in hippocampus].Sheng Li Xue Bao. 2016 Jun 25;68(3):335-42. Sheng Li Xue Bao. 2016. PMID: 27350206 Review. Chinese.
-
Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory.J Neurosci. 2016 Nov 23;36(47):11851-11864. doi: 10.1523/JNEUROSCI.1700-16.2016. J Neurosci. 2016. PMID: 27881773 Free PMC article.
-
Role of synaptic structural plasticity in impairments of spatial learning and memory induced by developmental lead exposure in Wistar rats.PLoS One. 2014 Dec 23;9(12):e115556. doi: 10.1371/journal.pone.0115556. eCollection 2014. PLoS One. 2014. PMID: 25536363 Free PMC article.
-
Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity.Neurotoxicology. 2000 Dec;21(6):1057-68. Neurotoxicology. 2000. PMID: 11233752 Review.
Cited by
-
Lead-exposure associated miRNAs in humans and Alzheimer's disease as potential biomarkers of the disease and disease processes.Sci Rep. 2022 Sep 24;12(1):15966. doi: 10.1038/s41598-022-20305-5. Sci Rep. 2022. PMID: 36153426 Free PMC article.
-
Building a Network of Adverse Outcome Pathways (AOPs) Incorporating the Tau-Driven AOP Toward Memory Loss (AOP429).J Alzheimers Dis Rep. 2022 Jun 7;6(1):271-296. doi: 10.3233/ADR-220015. eCollection 2022. J Alzheimers Dis Rep. 2022. PMID: 35891639 Free PMC article. Review.
-
Spirulina as a natural shield: a comprehensive review of its protective effects against lead toxicity.Arch Toxicol. 2025 Jul 2. doi: 10.1007/s00204-025-04113-0. Online ahead of print. Arch Toxicol. 2025. PMID: 40600982 Review.
-
The neurophysiological consequences of racism-related stressors in Black Americans.Neurosci Biobehav Rev. 2024 Jun;161:105638. doi: 10.1016/j.neubiorev.2024.105638. Epub 2024 Mar 24. Neurosci Biobehav Rev. 2024. PMID: 38522814 Free PMC article. Review.
-
Genetic deletion of zinc transporter ZnT3 induces progressive cognitive deficits in mice by impairing dendritic spine plasticity and glucose metabolism.Front Mol Neurosci. 2024 May 14;17:1375925. doi: 10.3389/fnmol.2024.1375925. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38807922 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

