Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review
- PMID: 33718398
- PMCID: PMC7952971
- DOI: 10.3389/fmed.2021.595371
Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review
Abstract
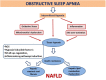
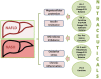
Non-alcoholic fatty liver disease (NAFLD) is a challenging disease caused by multiple factors, which may partly explain why it remains still orphan of an adequate therapeutic strategy. Herein we focus on the interplay between oxidative stress (OS) and the other causal pathogenetic factors. Different reactive oxygen species (ROS) generators contribute to NAFLD inflammatory and fibrotic progression, which is quite strictly linked to the lipotoxic liver injury from fatty acids and/or a wide variety of their biologically active metabolites in the context of either a two-hit or a (more recent) multiple parallel hits theory. An antioxidant defense system is usually able to protect hepatic cells from damaging effects caused by ROS, including those produced into the gastrointestinal tract, i.e., by-products generated by usual cellular metabolic processes, normal or dysbiotic microbiota, and/or diet through an enhanced gut-liver axis. Oxidative stress originating from the imbalance between ROS generation and antioxidant defenses is under the influence of individual genetic and epigenetic factors as well. Healthy diet and physical activity have been shown to be effective on NAFLD also with antioxidant mechanisms, but compliance to these lifestyles is very low. Among several considered antioxidants, vitamin E has been particularly studied; however, data are still contradictory. Some studies with natural polyphenols proposed for NAFLD prevention and treatment are encouraging. Probiotics, prebiotics, diet, or fecal microbiota transplantation represent new therapeutic approaches targeting the gut microbiota dysbiosis. In the near future, precision medicine taking into consideration genetic or environmental epigenetic risk factors will likely assist in further selecting the treatment that could work best for a specific patient.
Keywords: antioxidants; gut microbiota; metabolic syndrome; non-alcoholic fatty liver disease; obesity; obstructive sleep apnea syndrome; oxidative stress.
Copyright © 2021 Delli Bovi, Marciano, Mandato, Siano, Savoia and Vajro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. (1980) 55:434–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

