Performance of Saliva Samples for COVID-19 Diagnosis by Using the AllplexTM 2019-nCoV Assay Kit
- PMID: 33718401
- PMCID: PMC7943474
- DOI: 10.3389/fmed.2021.617399
Performance of Saliva Samples for COVID-19 Diagnosis by Using the AllplexTM 2019-nCoV Assay Kit
Abstract
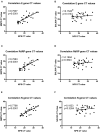
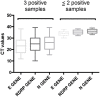
Background: Although the nasopharyngeal swab (NPS) is the reference sampling method for the detection of SARS-Cov-2, it is not always possible to collect NPS in some patients. Saliva represents an interesting sampling method because it is less invasive and more convenient in patients with nasal or pharyngeal lesions. Objective: To compare the RT-qPCR test performances of saliva samples with nasal mid-turbinate swab (NMTS) and NPS samples in a cohort of ambulatory patients suspected of having COVID-19. Study Design: For each of the 112 enrolled patients, NPS, NMTS, and saliva samples were collected and tested for SARS-Cov-2 detection using three different target genes (RdRP, N and E genes) by RT-qPCR. Results: Among the positive samples (56/112), saliva samples showed a lower percentage of SARS-Cov-2 detection compared to NPS samples, (85.7 vs. 96.4%), while still a lower percentage was observed for NMTS samples (78.6%). In average, saliva samples showed higher Ct values for all tested target genes, compared to those from NPS and NMTS samples. Conclusions: By using the AllplexTM 2019-nCoV Assay Kit, saliva samples showed lower sensitivity for SARS CoV-2 compared to NPS samples; however, the not detected cases had lower viral burden in NPS samples (CT values >33) representing an interesting alternative sampling method in patients in which it is not possible to take a NPS sample.
Keywords: COVID-19; RT-qPCR; SAR-CoV-2; nasopharangeal swabs; saliva.
Copyright © 2021 Tapia, Marcia, Ivone, Nadia, Lesly, Camila, Valentina, Paula and Fabien.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Centers for Disease Control and prevention (CDC) . Interim Guidelines for Clinical Specimens for COVID-19. Centers Dis Control Prev (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
-
- World Health Organization (WHO) . Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases:Interim Guidance. World Heal Organ (2020). Available online at: https://apps.who.int/iris/handle/10665/331329
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

