Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-infarction LV Remodeling
- PMID: 33718449
- PMCID: PMC7952323
- DOI: 10.3389/fcvm.2021.621781
Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-infarction LV Remodeling
Abstract
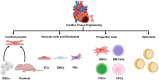
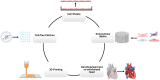
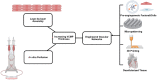
Tissue engineering combines principles of engineering and biology to generate living tissue equivalents for drug testing, disease modeling, and regenerative medicine. As techniques for reprogramming human somatic cells into induced pluripotent stem cells (iPSCs) and subsequently differentiating them into cardiomyocytes and other cardiac cells have become increasingly efficient, progress toward the development of engineered human cardiac muscle patch (hCMP) and heart tissue analogs has accelerated. A few pilot clinical studies in patients with post-infarction LV remodeling have been already approved. Conventional methods for hCMP fabrication include suspending cells within scaffolds, consisting of biocompatible materials, or growing two-dimensional sheets that can be stacked to form multilayered constructs. More recently, advanced technologies, such as micropatterning and three-dimensional bioprinting, have enabled fabrication of hCMP architectures at unprecedented spatiotemporal resolution. However, the studies working on various hCMP-based strategies for in vivo tissue repair face several major obstacles, including the inadequate scalability for clinical applications, poor integration and engraftment rate, and the lack of functional vasculature. Here, we review many of the recent advancements and key concerns in cardiac tissue engineering, focusing primarily on the production of hCMPs at clinical/industrial scales that are suitable for administration to patients with myocardial disease. The wide variety of cardiac cell types and sources that are applicable to hCMP biomanufacturing are elaborated. Finally, some of the key challenges remaining in the field and potential future directions to address these obstacles are discussed.
Keywords: cardiac patch; cardiac regeneration and remodeling; heart failure; myocardial infarction; myocardium; regenerative medicine; tissue engineering.
Copyright © 2021 Wang, Serpooshan and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Advances in Cardiac Tissue Engineering.Bioengineering (Basel). 2022 Nov 16;9(11):696. doi: 10.3390/bioengineering9110696. Bioengineering (Basel). 2022. PMID: 36421097 Free PMC article. Review.
-
Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine.Circulation. 2018 Apr 17;137(16):1712-1730. doi: 10.1161/CIRCULATIONAHA.117.030785. Epub 2017 Dec 12. Circulation. 2018. PMID: 29233823 Free PMC article.
-
Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold.Circ Res. 2017 Apr 14;120(8):1318-1325. doi: 10.1161/CIRCRESAHA.116.310277. Epub 2017 Jan 9. Circ Res. 2017. PMID: 28069694 Free PMC article.
-
Layer-By-Layer Fabrication of Thicker and Larger Human Cardiac Muscle Patches for Cardiac Repair in Mice.Front Cardiovasc Med. 2022 Jan 6;8:800667. doi: 10.3389/fcvm.2021.800667. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35071364 Free PMC article.
-
Three-dimensional scaffold-free microtissues engineered for cardiac repair.J Mater Chem B. 2020 Sep 14;8(34):7571-7590. doi: 10.1039/d0tb01528h. Epub 2020 Jul 29. J Mater Chem B. 2020. PMID: 32724973 Free PMC article. Review.
Cited by
-
In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between.Biomedicines. 2024 Nov 27;12(12):2714. doi: 10.3390/biomedicines12122714. Biomedicines. 2024. PMID: 39767621 Free PMC article. Review.
-
Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches.Drug Deliv Transl Res. 2023 Jul;13(7):1983-2014. doi: 10.1007/s13346-023-01290-2. Epub 2023 Feb 10. Drug Deliv Transl Res. 2023. PMID: 36763330 Free PMC article. Review.
-
The Exciting Realities and Possibilities of iPS-Derived Cardiomyocytes.Bioengineering (Basel). 2023 Feb 10;10(2):237. doi: 10.3390/bioengineering10020237. Bioengineering (Basel). 2023. PMID: 36829731 Free PMC article. Review.
-
FSTL-1 loaded 3D bioprinted vascular patch regenerates the ischemic heart tissue.iScience. 2024 Aug 22;27(10):110770. doi: 10.1016/j.isci.2024.110770. eCollection 2024 Oct 18. iScience. 2024. PMID: 39398249 Free PMC article.
-
Advances in Cardiac Tissue Engineering.Bioengineering (Basel). 2022 Nov 16;9(11):696. doi: 10.3390/bioengineering9110696. Bioengineering (Basel). 2022. PMID: 36421097 Free PMC article. Review.
References
-
- Laslett LJ, Alagona P, Jr., Clark BA, III, Drozda JP, Saldivar F, Wilson SR, et al. . The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. (2012) 60(Suppl. 25):S1–49. 10.1016/j.jacc.2012.11.002 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

