Thioredoxin 1 (TRX1) Overexpression Cancels the Slow Force Response (SFR) Development
- PMID: 33718450
- PMCID: PMC7952880
- DOI: 10.3389/fcvm.2021.622583
Thioredoxin 1 (TRX1) Overexpression Cancels the Slow Force Response (SFR) Development
Abstract
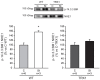
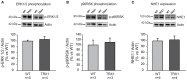
The stretch of cardiac muscle increases developed force in two phases. The first phase occurs immediately after stretch and is the expression of the Frank-Starling mechanism, while the second one or slow force response (SFR) occurs gradually and is due to an increase in the calcium transient amplitude. An important step in the chain of events leading to the SFR generation is the increased production of reactive oxygen species (ROS) leading to redox sensitive ERK1/2, p90RSK, and NHE1 phosphorylation/activation. Conversely, suppression of ROS production blunts the SFR. The purpose of this study was to explore whether overexpression of the ubiquitously expressed antioxidant molecule thioredoxin-1 (TRX1) affects the SFR development and NHE1 phosphorylation. We did not detect any change in basal phopho-ERK1/2, phopho-p90RSK, and NHE1 expression in mice with TRX1 overexpression compared to wild type (WT). Isolated papillary muscles from WT or TRX1-overexpressing mice were stretched from 92 to 98% of its maximal length. A prominent SFR was observed in WT mice that was completely canceled in TRX1 animals. Interestingly, myocardial stretch induced a significant increase in NHE1 phosphorylation in WT mice that was not detected in TRX1-overexpressing mice. These novel results suggest that magnification of cardiac antioxidant defense power by overexpression of TRX1 precludes NHE1 phosphorylation/activation after stretch, consequently blunting the SFR development.
Keywords: NHE1; SFR; TRX1; antioxidant; cardiac hypertrophy.
Copyright © 2021 Zavala, Díaz, Villa-Abrille and Pérez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
p38-MAP Kinase Negatively Regulates the Slow Force Response to Stretch in Rat Myocardium through the Up-Regulation of Dual Specificity Phosphatase 6 (DUSP6).Cell Physiol Biochem. 2019;52(2):172-185. doi: 10.33594/000000012. Epub 2019 Feb 28. Cell Physiol Biochem. 2019. PMID: 30816666
-
Inhibition of carbonic anhydrase prevents the Na(+)/H(+) exchanger 1-dependent slow force response to rat myocardial stretch.Am J Physiol Heart Circ Physiol. 2013 Jul 15;305(2):H228-37. doi: 10.1152/ajpheart.00055.2013. Epub 2013 May 24. Am J Physiol Heart Circ Physiol. 2013. PMID: 23709596
-
Mitochondrial reactive oxygen species activate the slow force response to stretch in feline myocardium.J Physiol. 2007 Nov 1;584(Pt 3):895-905. doi: 10.1113/jphysiol.2007.141689. Epub 2007 Sep 6. J Physiol. 2007. PMID: 17823205 Free PMC article.
-
The autocrine/paracrine loop after myocardial stretch: mineralocorticoid receptor activation.Curr Cardiol Rev. 2013 Aug;9(3):230-40. doi: 10.2174/1573403x113099990034. Curr Cardiol Rev. 2013. PMID: 23909633 Free PMC article. Review.
-
Stretch-induced Slow Force Response in Mammalian Ventricular Myocardium.In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. PMID: 21290760 Free Books & Documents. Review.
Cited by
-
Identification of thioredoxin-1 as a biomarker of lung cancer and evaluation of its prognostic value based on bioinformatics analysis.Front Oncol. 2023 Jan 27;13:1080237. doi: 10.3389/fonc.2023.1080237. eCollection 2023. Front Oncol. 2023. PMID: 36776308 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

