Effective, safe, and sustained correction of murine XLA using a UCOE-BTK promoter-based lentiviral vector
- PMID: 33718514
- PMCID: PMC7907679
- DOI: 10.1016/j.omtm.2021.01.007
Effective, safe, and sustained correction of murine XLA using a UCOE-BTK promoter-based lentiviral vector
Abstract
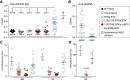
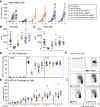
X-linked agammaglobulinemia (XLA) is an immune disorder caused by mutations in Bruton's tyrosine kinase (BTK). BTK is expressed in B and myeloid cells, and its deficiency results in a lack of mature B cells and protective antibodies. We previously reported a lentivirus (LV) BTK replacement therapy that restored B cell development and function in Btk and Tec double knockout mice (a phenocopy of human XLA). In this study, with the goal of optimizing both the level and lineage specificity of BTK expression, we generated LV incorporating the proximal human BTK promoter. Hematopoietic stem cells from Btk -/- Tec -/- mice transduced with this vector rescued lineage-specific expression and restored B cell function in Btk -/- Tec -/- recipients. Next, we tested addition of candidate enhancers and/or ubiquitous chromatin opening elements (UCOEs), as well as codon optimization to improve BTK expression. An Eμ enhancer improved B cell rescue, but increased immunoglobulin G (IgG) autoantibodies. Addition of the UCOE avoided autoantibody generation while improving B cell development and function and reducing vector silencing. An optimized vector containing a truncated UCOE upstream of the BTK promoter and codon-optimized BTK cDNA resulted in stable, lineage-regulated BTK expression that mirrored endogenous BTK, making it a strong candidate for XLA therapy.
Keywords: BTK; Bruton's tyrosine kinase; DNase hypersensitive sites; UCOE; X-linked Agammaglobulinemia; XLA; codon optimization; gene therapy; hematopoietic stem cells; lentivirus; primary immunodeficiency.
© 2021 The Author(s).
Conflict of interest statement
D.J.R., S.S., and K.S. are inventors on a patent filed by Seattle Children’s Research Institute related to the lentiviral vectors described in this study. The remaining authors declare no competing interests.
Figures






Similar articles
-
B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia.Blood. 2010 Mar 18;115(11):2146-55. doi: 10.1182/blood-2009-09-241869. Epub 2010 Jan 21. Blood. 2010. PMID: 20093406 Free PMC article.
-
Sustained correction of B-cell development and function in a murine model of X-linked agammaglobulinemia (XLA) using retroviral-mediated gene transfer.Blood. 2004 Sep 1;104(5):1281-90. doi: 10.1182/blood-2003-09-3044. Epub 2004 May 13. Blood. 2004. PMID: 15142874
-
Correction of B-cell development in Btk-deficient mice using lentiviral vectors with codon-optimized human BTK.Leukemia. 2010 Sep;24(9):1617-30. doi: 10.1038/leu.2010.140. Epub 2010 Jun 24. Leukemia. 2010. PMID: 20574453
-
Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling.Immunol Rev. 2005 Feb;203:200-15. doi: 10.1111/j.0105-2896.2005.00225.x. Immunol Rev. 2005. PMID: 15661031 Review.
-
Bruton tyrosine kinase (BTK) in X-linked agammaglobulinemia (XLA).Front Biosci. 2000 Dec 1;5:D917-28. doi: 10.2741/vihinen. Front Biosci. 2000. PMID: 11102316 Review.
Cited by
-
A Registry Study of 240 Patients with X-Linked Agammaglobulinemia Living in the USA.J Clin Immunol. 2023 Aug;43(6):1468-1477. doi: 10.1007/s10875-023-01502-x. Epub 2023 May 23. J Clin Immunol. 2023. PMID: 37219739 Free PMC article.
-
The use of RNA-based treatments in the field of cancer immunotherapy.Mol Cancer. 2023 Jul 7;22(1):106. doi: 10.1186/s12943-023-01807-w. Mol Cancer. 2023. PMID: 37420174 Free PMC article. Review.
-
Future Drug Targets in Periodontal Personalised Medicine-A Narrative Review.J Pers Med. 2022 Feb 28;12(3):371. doi: 10.3390/jpm12030371. J Pers Med. 2022. PMID: 35330371 Free PMC article. Review.
-
Hematopoietic stem cell gene editing rescues B-cell development in X-linked agammaglobulinemia.J Allergy Clin Immunol. 2024 Jul;154(1):195-208.e8. doi: 10.1016/j.jaci.2024.03.003. Epub 2024 Mar 11. J Allergy Clin Immunol. 2024. PMID: 38479630 Free PMC article.
-
Advances in gene therapy for inborn errors of immunity.Curr Opin Allergy Clin Immunol. 2023 Dec 1;23(6):467-477. doi: 10.1097/ACI.0000000000000952. Epub 2023 Oct 13. Curr Opin Allergy Clin Immunol. 2023. PMID: 37846903 Free PMC article. Review.
References
-
- Brosens L.A., Tytgat K.M., Morsink F.H., Sinke R.J., Ten Berge I.J., Giardiello F.M., Offerhaus G.J., Keller J.J. Multiple colorectal neoplasms in X-linked agammaglobulinemia. Clin. Gastroenterol. Hepatol. 2008;6:115–119. - PubMed
-
- Lee P.P., Chen T.X., Jiang L.P., Chan K.W., Yang W., Lee B.W., Chiang W.C., Chen X.Y., Fok S.F., Lee T.L. Clinical characteristics and genotype-phenotype correlation in 62 patients with X-linked agammaglobulinemia. J. Clin. Immunol. 2010;30:121–131. - PubMed
-
- Plebani A., Soresina A., Rondelli R., Amato G.M., Azzari C., Cardinale F., Cazzola G., Consolini R., De Mattia D., Dell’Erba G., Italian Pediatric Group for XLA-AIEOP Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin. Immunol. 2002;104:221–230. - PubMed
-
- Misbah S.A., Spickett G.P., Ryba P.C., Hockaday J.M., Kroll J.S., Sherwood C., Kurtz J.B., Moxon E.R., Chapel H.M. Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J. Clin. Immunol. 1992;12:266–270. - PubMed
-
- Hermaszewski R.A., Webster A.D. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q. J. Med. 1993;86:31–42. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

