Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform
- PMID: 33718594
- PMCID: PMC7917457
- DOI: 10.1016/j.omto.2021.02.006
Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform
Abstract
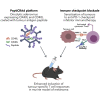
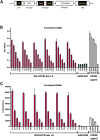
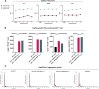
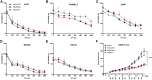
Oncolytic viruses (OVs) have been shown to induce anti-cancer immunity and enhance cancer immunotherapies, such as immune checkpoint inhibitor therapies. OV therapies can be further improved by arming OVs with immunostimulatory molecules, including various cytokines or chemokines. Here, we have developed a novel adenovirus encoding two immunostimulatory molecules: cluster of differentiation 40 ligand (CD40L) and tumor necrosis factor receptor superfamily member 4 ligand (OX40L). This novel virus, designated VALO-D102, is designed to activate both innate and adaptive immune responses against tumors. CD40L affects the innate side by licensing antigen-presenting cells to drive CD8+ T cell responses, and OX40L increases clonal expansion and survival of CD8+ T cells and formation of a larger pool of memory T cells. VALO-D102 and its murine surrogate VALO-mD901, expressing murine OX40L and CD40L, were used in our previously developed PeptiCRAd cancer vaccine platform. Intratumoral administration of PeptiCRAd significantly increased tumor-specific T cell responses, reduced tumor growth, and induced systemic anti-cancer immunity in two mouse models of melanoma. In addition, PeptiCRAd therapy, in combination with anti-PD-1 immune checkpoint inhibitor therapy, significantly improved tumor growth control as compared to either monotherapy alone.
Keywords: CD40L; OX40L; PeptiCRAd; T cell activation; oncolytic vaccine.
© 2021 The Authors.
Conflict of interest statement
V.C. is a cofounder and shareholder at Valo Therapeutics Oy. The other authors declare no competing interests.
Figures



Similar articles
-
Modulating Oncolytic Adenovirus Immunotherapy by Driving Two Axes of the Immune System by Expressing 4-1BBL and CD40L.Hum Gene Ther. 2022 Mar;33(5-6):250-261. doi: 10.1089/hum.2021.197. Epub 2022 Jan 6. Hum Gene Ther. 2022. PMID: 34731019 Free PMC article.
-
The adjuvancy of OX40 ligand (CD252) on an HIV-1 canarypox vaccine.Vaccine. 2009 Aug 13;27(37):5077-84. doi: 10.1016/j.vaccine.2009.06.046. Epub 2009 Jun 30. Vaccine. 2009. PMID: 19573639
-
Systemic immunity upon local oncolytic virotherapy armed with immunostimulatory genes may be supported by tumor-derived exosomes.Mol Ther Oncolytics. 2021 Feb 17;20:508-518. doi: 10.1016/j.omto.2021.02.007. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33738337 Free PMC article.
-
Design and application of oncolytic viruses for cancer immunotherapy.Curr Opin Biotechnol. 2020 Oct;65:25-36. doi: 10.1016/j.copbio.2019.11.016. Epub 2019 Dec 23. Curr Opin Biotechnol. 2020. PMID: 31874424 Review.
-
Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy.Cells. 2020 Feb 10;9(2):400. doi: 10.3390/cells9020400. Cells. 2020. PMID: 32050597 Free PMC article. Review.
Cited by
-
Novel combinatorial therapy of oncolytic adenovirus AdV5/3-D24-ICOSL-CD40L with anti PD-1 exhibits enhanced anti-cancer efficacy through promotion of intratumoral T-cell infiltration and modulation of tumour microenvironment in mesothelioma mouse model.Front Oncol. 2023 Nov 20;13:1259314. doi: 10.3389/fonc.2023.1259314. eCollection 2023. Front Oncol. 2023. PMID: 38053658 Free PMC article.
-
Virus nanotechnology for intratumoural immunotherapy.Nat Rev Bioeng. 2024 Nov;2(11):916-929. doi: 10.1038/s44222-024-00231-z. Epub 2024 Sep 23. Nat Rev Bioeng. 2024. PMID: 39698315 Free PMC article.
-
Enhanced durability of a Zika virus self-amplifying RNA vaccine through combinatorial OX40 and 4-1BB agonism.JCI Insight. 2025 Apr 3;10(10):e187405. doi: 10.1172/jci.insight.187405. eCollection 2025 May 22. JCI Insight. 2025. PMID: 40178907 Free PMC article.
-
Translational Aspects of Epithelioid Sarcoma: Current Consensus.Clin Cancer Res. 2024 Mar 15;30(6):1079-1092. doi: 10.1158/1078-0432.CCR-23-2174. Clin Cancer Res. 2024. PMID: 37916971 Free PMC article. Review.
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
References
-
- Ku G.Y., Yuan J., Page D.B., Schroeder S.E., Panageas K.S., Carvajal R.D., Chapman P.B., Schwartz G.K., Allison J.P., Wolchok J.D. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. - PMC - PubMed
-
- Yuan J., Adamow M., Ginsberg B.A., Rasalan T.S., Ritter E., Gallardo H.F., Xu Y., Pogoriler E., Terzulli S.L., Kuk D. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc. Natl. Acad. Sci. USA. 2011;108:16723–16728. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

