A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings
- PMID: 33718665
- PMCID: PMC7905459
- DOI: 10.1016/j.bioactmat.2021.01.039
A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings
Abstract
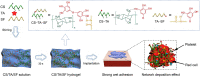
In recent years, the developed hemostatic technologies are still difficult to be applied to the hemostasis of massive arterial and visceral hemorrhage, owing to their weak hemostatic function, inferior wet tissue adhesion, and low mechanical properties. Herein, a mussel-inspired supramolecular interaction-cross-linked hydrogel with robust mechanical property (308.47 ± 29.20 kPa) and excellent hemostatic efficiency (96.5% ± 2.1%) was constructed as a hemostatic sealant. Typically, we combined chitosan (CS) with silk fibroin (SF) by cross-linking them through tannic acid (TA) to maintain the structural stability of the hydrogel, especially for wet tissue adhesion ability (shear adhesive strength = 29.66 ± 0.36 kPa). Compared with other materials reported previously, the obtained CS/TA/SF hydrogel yielded a lower amount of blood loss and shorter time to hemostasis in various arterial and visceral bleeding models, which could be ascribed to the synergistic effect of wound closure under wet state as well as intrinsic hemostatic activity of CS. As a superior hemostatic sealant, the unique hydrogel proposed in this work can be exploited to offer significant advantages in the acute wound and massive hemorrhage with the restrictive access of therapeutic moieties.
Keywords: Arterial and visceral bleeding models; Hemostatic sealant; Mussel-inspired hydrogel; Robust tissue anchor; Supramolecular cross-linking.
© 2021 [The Author/The Authors].
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Han W., Zhou B., Yang K., Xiong X., Luan S., Wang Y., Xu Z., Lei P., Luo Z., Gao J., Zhan Y., Chen G., Liang L., Wang R., Li S., Xu H. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020;5(4):768–778. - PMC - PubMed
-
- Wang A.Y., Rafalko J., MacDonald M., Ming X., Kocharian R. Absorbable hemostatic aggregates. ACS Biomater. Sci. Eng. 2017;3(12):3675–3686. - PubMed
-
- Arnaud F., Tomori T., Carr W., McKeague A., Teranishi K., Prusaczyk K., McCarron R. Exothermic reaction in zeolite hemostatic dressings: QuikClot ACS and ACS+®. Ann. Biomed. Eng. 2008;36(10):1708–1713. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

