Shedding Light on Miniaturized Dialysis Using MXene 2D Materials: A Computational Chemistry Approach
- PMID: 33718722
- PMCID: PMC7948252
- DOI: 10.1021/acsomega.0c06118
Shedding Light on Miniaturized Dialysis Using MXene 2D Materials: A Computational Chemistry Approach
Abstract
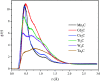
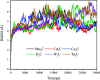
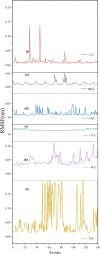
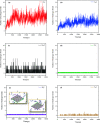
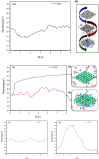
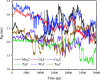
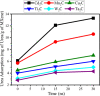
Materials science can pave the way toward developing novel devices at the service of human life. In recent years, computational materials engineering has been promising in predicting material performance prior to the experiments. Herein, this capability has been carefully employed to tackle severe problems associated with kidney diseases through proposing novel nanolayers to adsorb urea and accordingly causing the wearable artificial kidney (WAK) to be viable. The two-dimensional metal carbide and nitride (MXene) nanosheets can leverage the performance of various devices since they are highly tunable along with fascinating surface chemistry properties. In this study, molecular dynamics (MD) simulations were exploited to investigate the interactions between urea and different MXene nanosheets. To this end, detailed analyses were performed that clarify the suitability of these nanostructures in urea adsorption. The atomistic simulations were carried out on Mn2C, Cd2C, Cu2C, Ti2C, W2C, Ta2C, and urea to determine the most appropriate urea-removing adsorbent. It was found that Cd2C was more efficient followed by Mn2C, which can be effectively exploited in WAK devices at the service of human health.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Mn2+-Assisted DNA Oligonucleotide Adsorption on Ti2C MXene Nanosheets.Langmuir. 2019 Jul 30;35(30):9858-9866. doi: 10.1021/acs.langmuir.9b01810. Epub 2019 Jul 16. Langmuir. 2019. PMID: 31265783
-
MXene Sorbents for Removal of Urea from Dialysate: A Step toward the Wearable Artificial Kidney.ACS Nano. 2018 Oct 23;12(10):10518-10528. doi: 10.1021/acsnano.8b06494. Epub 2018 Oct 1. ACS Nano. 2018. PMID: 30257087
-
Loading Self-Assembly Siliceous Zeolites for Affordable Next-Generation Wearable Artificial Kidney Technology.ACS Nano. 2024 Nov 5;18(44):30388-30404. doi: 10.1021/acsnano.4c07594. Epub 2024 Oct 24. ACS Nano. 2024. PMID: 39448556
-
First-Principles Studies of Adsorptive Remediation of Water and Air Pollutants Using Two-Dimensional MXene Materials.Materials (Basel). 2018 Nov 14;11(11):2281. doi: 10.3390/ma11112281. Materials (Basel). 2018. PMID: 30441865 Free PMC article. Review.
-
Recent Advanced on the MXene-Organic Hybrids: Design, Synthesis, and Their Applications.Nanomaterials (Basel). 2021 Jan 11;11(1):166. doi: 10.3390/nano11010166. Nanomaterials (Basel). 2021. PMID: 33440847 Free PMC article. Review.
Cited by
-
Engineering of 2D nanomaterials to trap and kill SARS-CoV-2: a new insight from multi-microsecond atomistic simulations.Drug Deliv Transl Res. 2022 Jun;12(6):1408-1422. doi: 10.1007/s13346-021-01054-w. Epub 2021 Sep 3. Drug Deliv Transl Res. 2022. PMID: 34476766 Free PMC article.
-
Molecular insight into COF monolayers for urea sorption in artificial kidneys.Sci Rep. 2021 Jun 8;11(1):12085. doi: 10.1038/s41598-021-91617-1. Sci Rep. 2021. PMID: 34103625 Free PMC article.
-
β-Amyloid Targeting with Two-Dimensional Covalent Organic Frameworks: Multi-Scale In-Silico Dissection of Nano-Biointerface.Chembiochem. 2021 Jul 1;22(13):2306-2318. doi: 10.1002/cbic.202100075. Epub 2021 May 11. Chembiochem. 2021. PMID: 33884725 Free PMC article.
-
Portable, wearable and implantable artificial kidney systems: needs, opportunities and challenges.Nat Rev Nephrol. 2023 Aug;19(8):481-490. doi: 10.1038/s41581-023-00726-9. Epub 2023 Jun 5. Nat Rev Nephrol. 2023. PMID: 37277461 Free PMC article. Review.
-
MXene-based electrochemical devices applied for healthcare applications.Mikrochim Acta. 2024 Jan 11;191(2):88. doi: 10.1007/s00604-023-06163-6. Mikrochim Acta. 2024. PMID: 38206460 Free PMC article. Review.
References
-
- van Gelder M. K.; Jong J. A.; Folkertsma L.; Guo Y.; Blüchel C.; Verhaar M. C.; Odijk M.; Van Nostrum C. F.; Hennink W. E.; Gerritsen K. G. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 11973510.1016/j.biomaterials.2019.119735. - DOI - PubMed
-
- Evangelidis N.; Tong A.; Manns B.; Hemmelgarn B.; Wheeler D. C.; Tugwell P.; Crowe S.; Harris T.; Van Biesen W.; Winkelmayer W. C.; Sautenet B.; et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am. J. Kidney Dis. 2017, 70, 464–475. 10.1053/j.ajkd.2016.11.029. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

