Affinity biosensors developed with quantum dots in microfluidic systems
- PMID: 33718778
- PMCID: PMC7944724
- DOI: 10.1007/s42247-021-00195-5
Affinity biosensors developed with quantum dots in microfluidic systems
Abstract
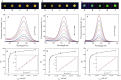
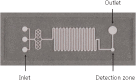
Quantum dots (QDs) are synthetic semiconductor nanocrystals with unique optical and electronic properties due to their size (2-10 nm) such as high molar absorption coefficient (10-100 times higher than organic dyes), resistance to chemical degradation, and unique optoelectronic properties due to quantum confinement (high quantum yield, emission color change with size). Compared to organic fluorophores, the narrower emission band and wider absorption bands of QDs offer great advantages in cell imaging and biosensor applications. The optoelectronic features of QDs have prompted their intensive use in bioanalytical, biophysical, and biomedical research. As the nanomaterials have been integrated into microfluidic systems, microfluidic technology has accelerated the adaptation of nanomaterials to clinical evaluation together with the advantages such as being more economical, more reproducible, and more susceptible to modification and integration with other technologies. Microfluidic systems serve an important role by being a platform in which QDs are integrated for biosensing applications. As we combine the advantages of QDs and microfluidic technology for biosensing technology, QD-based biosensor integrated with microfluidic systems can be used as an advanced and versatile diagnostic technology in case of pandemic. Specifically, there is an urgent necessity to have reliable and fast detection systems for COVID-19 virus. In this review, affinity-based biosensing mechanisms which are developed with QDs are examined in the domain of microfluidic approach. The combination of microfluidic technology and QD-based affinity biosensors are presented with examples in order to develop a better technological framework of diagnostic for COVID-19 virus.
Keywords: Biosensors; Microfluidic systems; Quantum dots.
© Qatar University and Springer Nature Switzerland AG 2021.
Figures










References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
