Impact of Pharmacogenomic Information on Values of Care and Quality of Life Associated with Codeine and Tramadol-Related Adverse Drug Events
- PMID: 33718782
- PMCID: PMC7930862
- DOI: 10.1016/j.mayocpiqo.2020.08.009
Impact of Pharmacogenomic Information on Values of Care and Quality of Life Associated with Codeine and Tramadol-Related Adverse Drug Events
Abstract
Objective: To assess the potential impact of Pharmacogenomic (PGx) variation in cytochrome P450 2D6 (CYP2D6) enzyme function, using loss in quality-adjusted life years (QALYs) associated with treatment problems, and the willingness to pay to avoid treatment problems from patients' and payers' perspectives.
Patients and methods: The study included patients prescribed tramadol or codeine, or both, between January 1, 2005, and December 31, 2017. Demographic information and adverse drug events, including adverse drug events and poor pain control, were collected from the electronic health records using natural language processing techniques and review by trained abstractors. Patients' willingness to pay and QALY estimates were based on comprehensive literature review. The CYP2D6 phenotypes were divided into 4 groups: ultra-rapid metabolizers, normal metabolizers, intermediate metabolizers, and poor metabolizers.
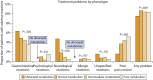
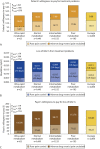
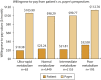
Results: Among the 2860 identified patients, 63 (2%) were ultrarapid metabolizers, 1449 (50%) were normal metabolizers, 1155 (40%) were intermediate metabolizers, and 193 (7%) were poor metabolizers. The patients' average estimated willingness-to-pay value to avoid treatment problems was $23 per month; poor metabolizers developed problems with the highest estimated willingness-to-pay value ($32 per month). The mean QALY loss among all patients was 0.024 QALYs (8.8 healthy days); poor metabolizers had the highest loss (0.027 QALYs, 9.9 healthy days).
Conclusion: Patients with various phenotypes developed different treatment problem profiles. Poor CYP2D6 metabolizers developed problems with highest willingness to pay, and they might potentially benefit most from PGx-guided treatment and problem prevention.
Keywords: ADE, adverse drug event; CYP2D6, Cytochrome P450 2D6; PGx, pharmacogenomics; QALY, quality-adjusted life year; REP, Rochester Epidemiology Project; RIGHT, Right Drug; Right Dose, Right Time-Using Genomic Data to Individualize Treatment.
© 2020 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc.
Figures
Similar articles
-
Description of Pharmacogenomic Testing Among Patients Admitted to the Intensive Care Unit After Cardiovascular Surgery.J Intensive Care Med. 2021 Nov;36(11):1281-1285. doi: 10.1177/0885066620946303. Epub 2020 Jul 31. J Intensive Care Med. 2021. PMID: 32734840
-
CYP2D6 phenotypes are associated with adverse outcomes related to opioid medications.Pharmgenomics Pers Med. 2017 Jul 24;10:217-227. doi: 10.2147/PGPM.S136341. eCollection 2017. Pharmgenomics Pers Med. 2017. PMID: 28769582 Free PMC article.
-
Sex Differences in Associations Between CYP2D6 Phenotypes and Response to Opioid Analgesics.Pharmgenomics Pers Med. 2020 Mar 13;13:71-79. doi: 10.2147/PGPM.S239222. eCollection 2020. Pharmgenomics Pers Med. 2020. PMID: 32214840 Free PMC article.
-
Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I.Clin Pharmacokinet. 2009;48(11):689-723. doi: 10.2165/11318030-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19817501 Review.
-
Influence of Cytochrome P450, Family 2, Subfamily D, Polypeptide 6 (CYP2D6) polymorphisms on pain sensitivity and clinical response to weak opioid analgesics.Drug Metab Pharmacokinet. 2014;29(1):29-43. doi: 10.2133/dmpk.dmpk-13-rv-032. Epub 2013 Jun 11. Drug Metab Pharmacokinet. 2014. PMID: 23759977 Review.
References
-
- Thorson D., Biewen P., Bonte B., et al. Health Care Protocol; 2014. Acute Pain Assessment and Opioid Prescribing Protocol. Bloomington, MN.
-
- Gruber C.M. Codeine phosphate, propoxyphene hydrochloride, and placebo. JAMA. 1957;164(9):966–969. - PubMed
-
- Beakley B.D., Kaye A.M., Kaye A.D. Tramadol, pharmacology, side effects, and serotonin syndrome: A review. Pain Physician. 2015;18(4):395–400. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

